Notes These are the class "big template" notes. Some "solves" are mixed in here.
Portions missing usually indicate things you do in class on slates, and were then advised to transfer to your notes.
Click on image to zoom in, right-click to save. (If you cannot interact with the image, try reloading.) All these are also present in your Google Folder under notes.
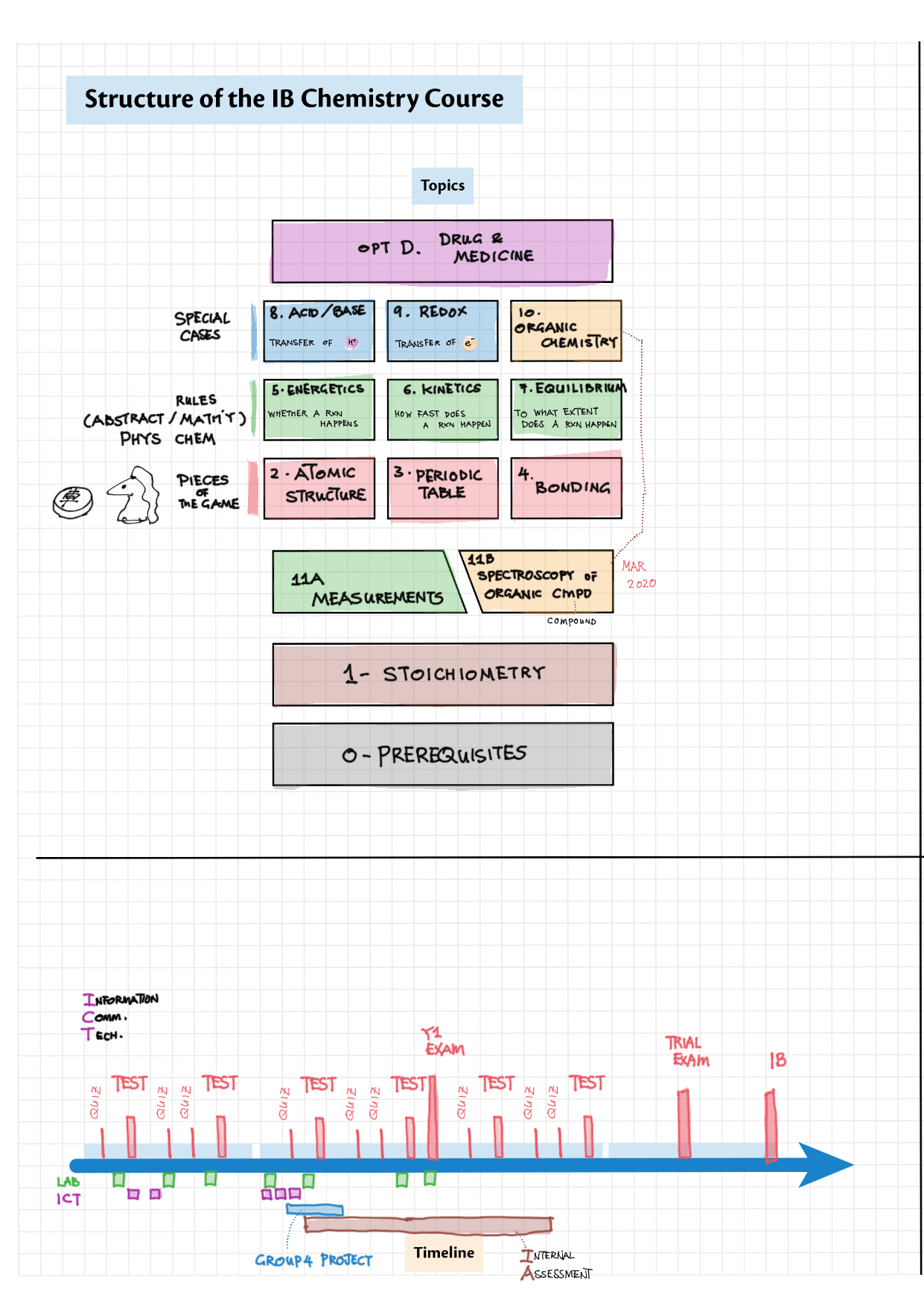
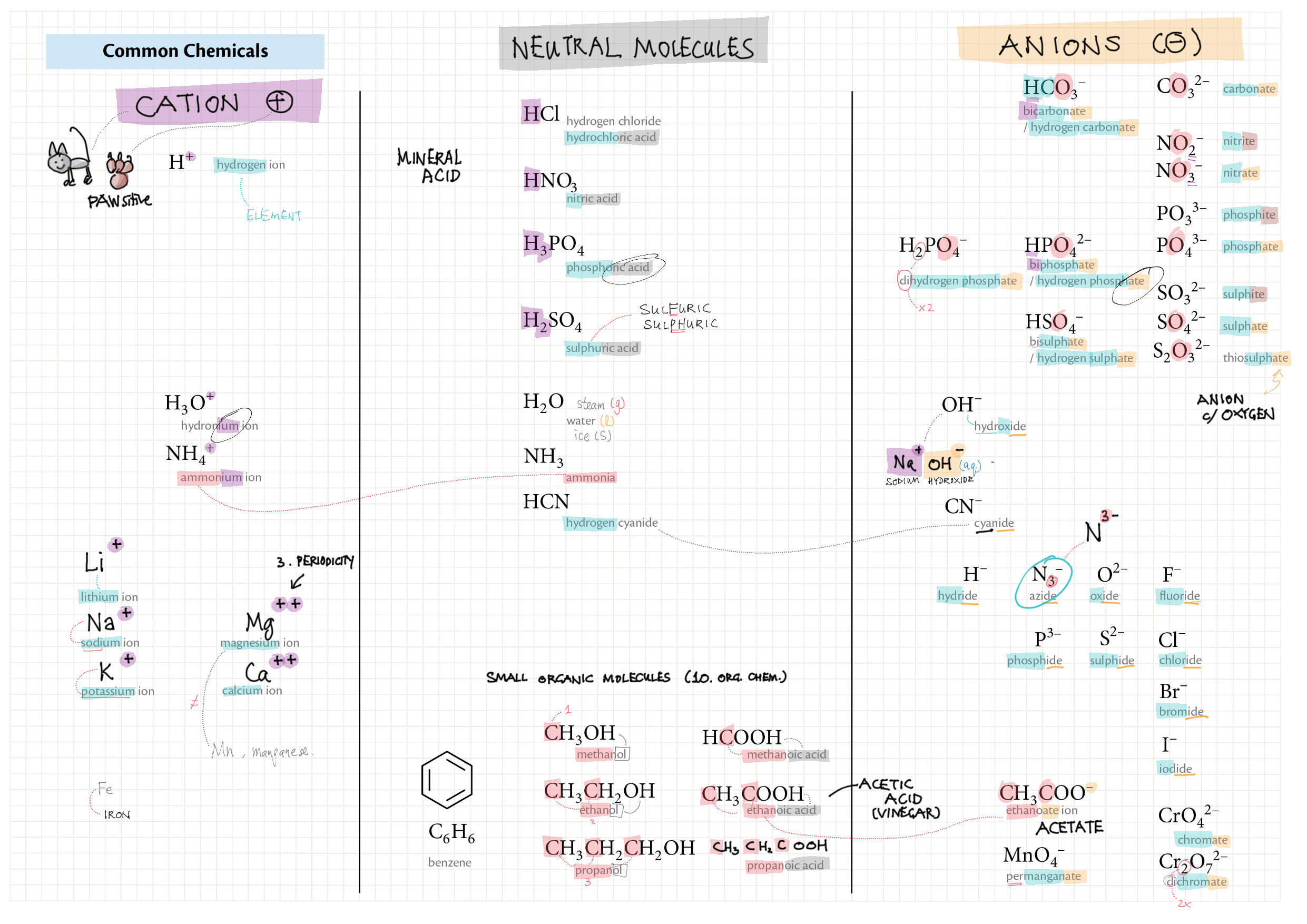
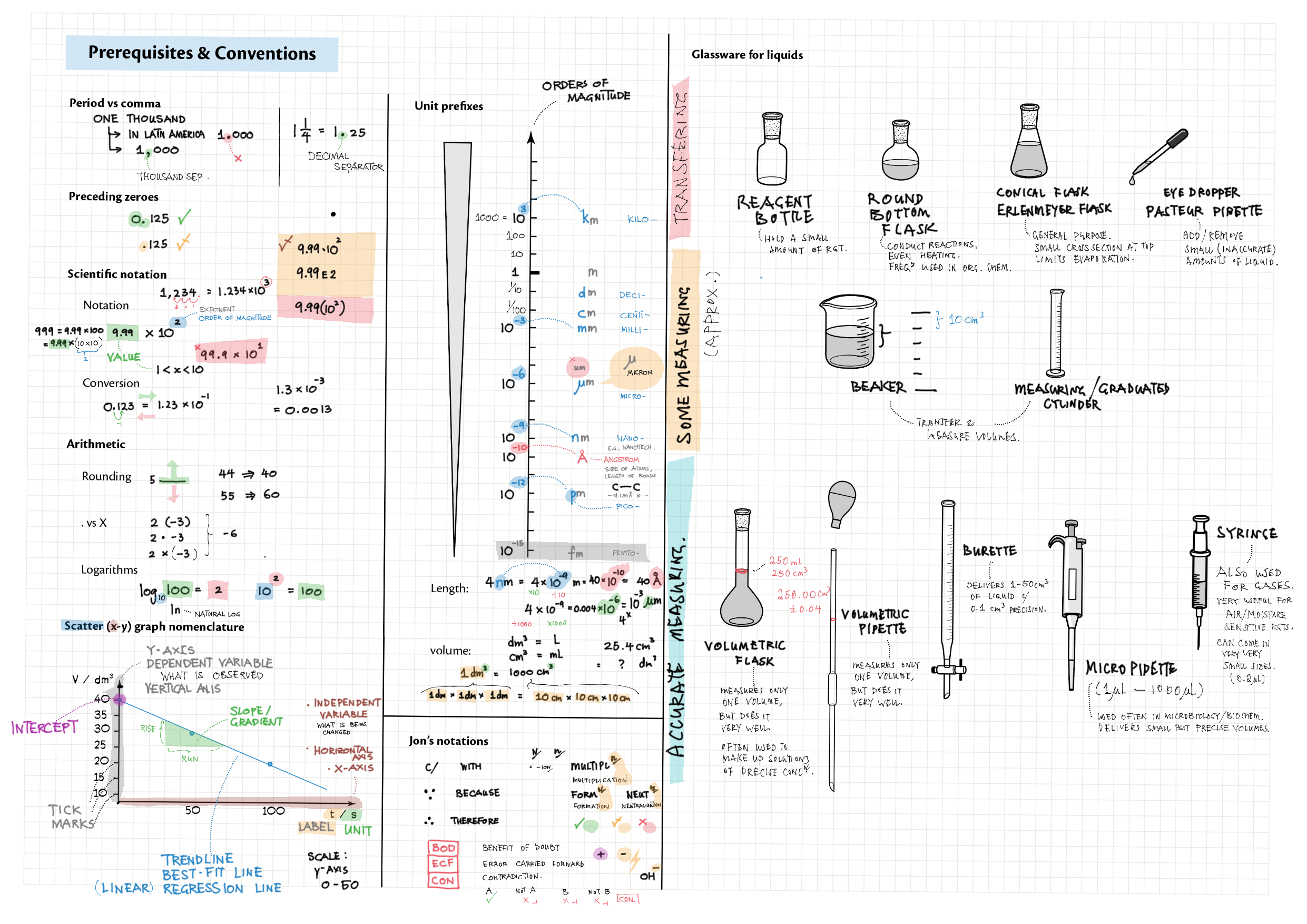
Zip (85 Mb) 0 - Prerequisites and Foundations topics note 0 Structure of IB Chemistry 0 Common chemicals 0 Prerequisites and conventions
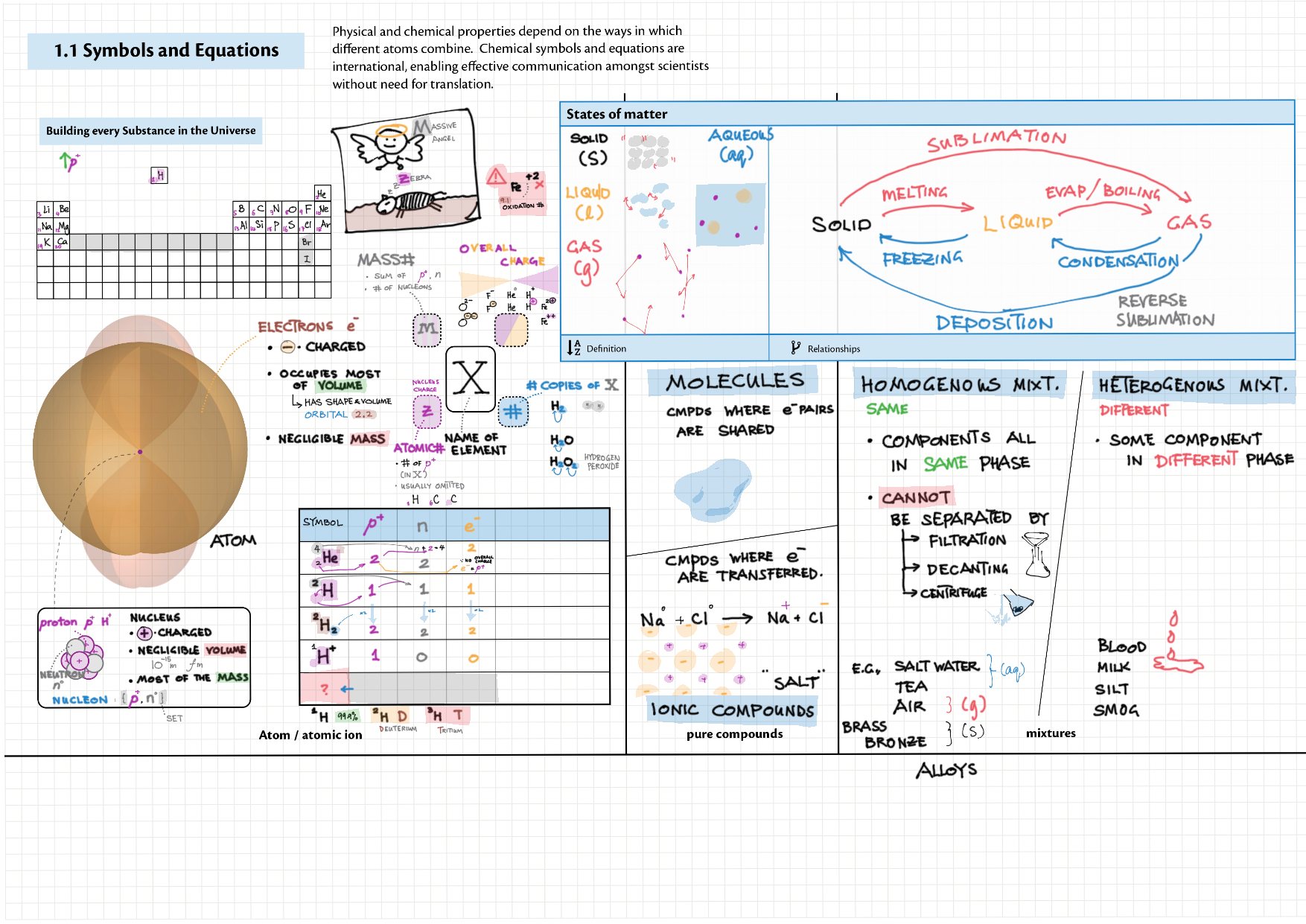
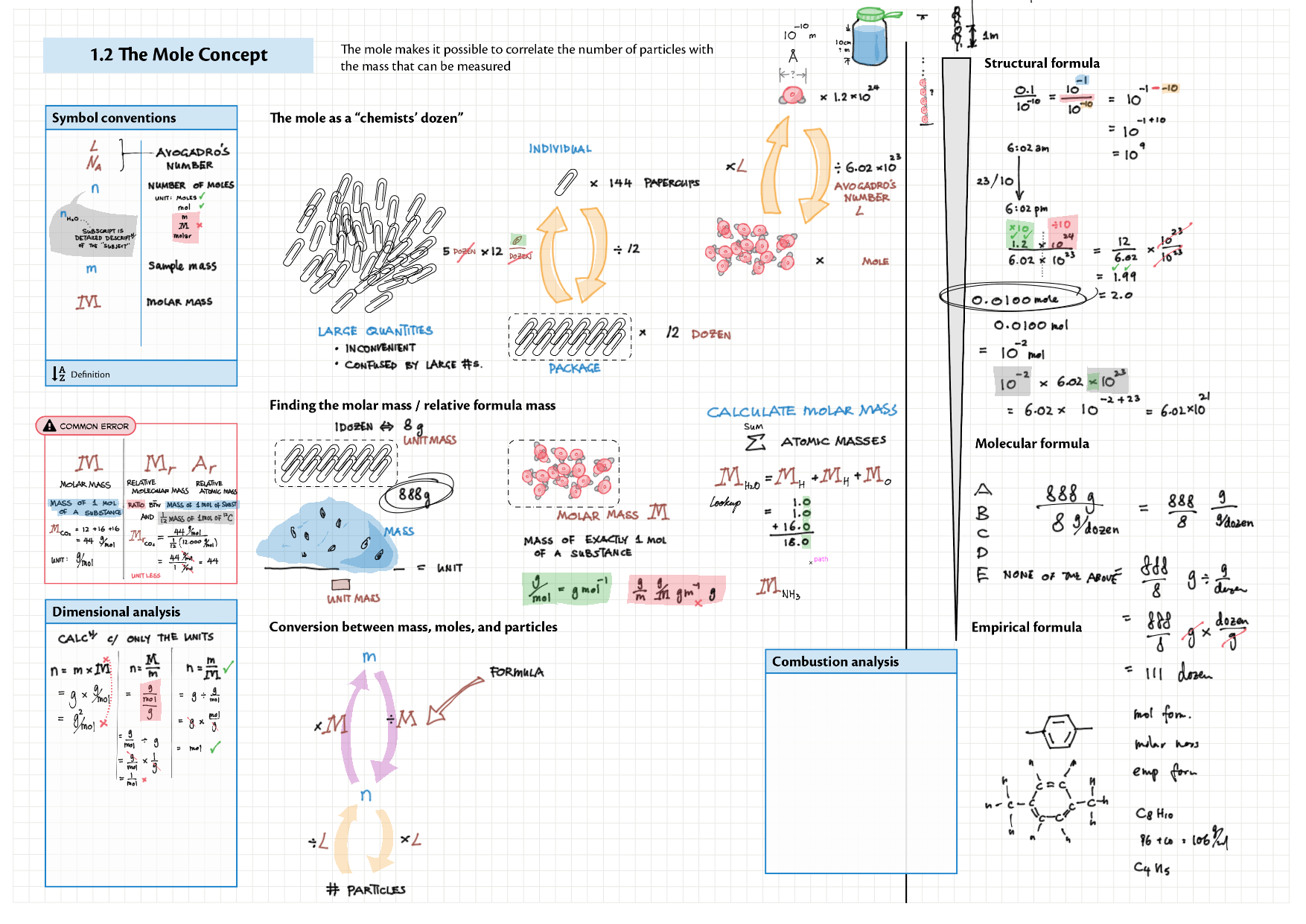
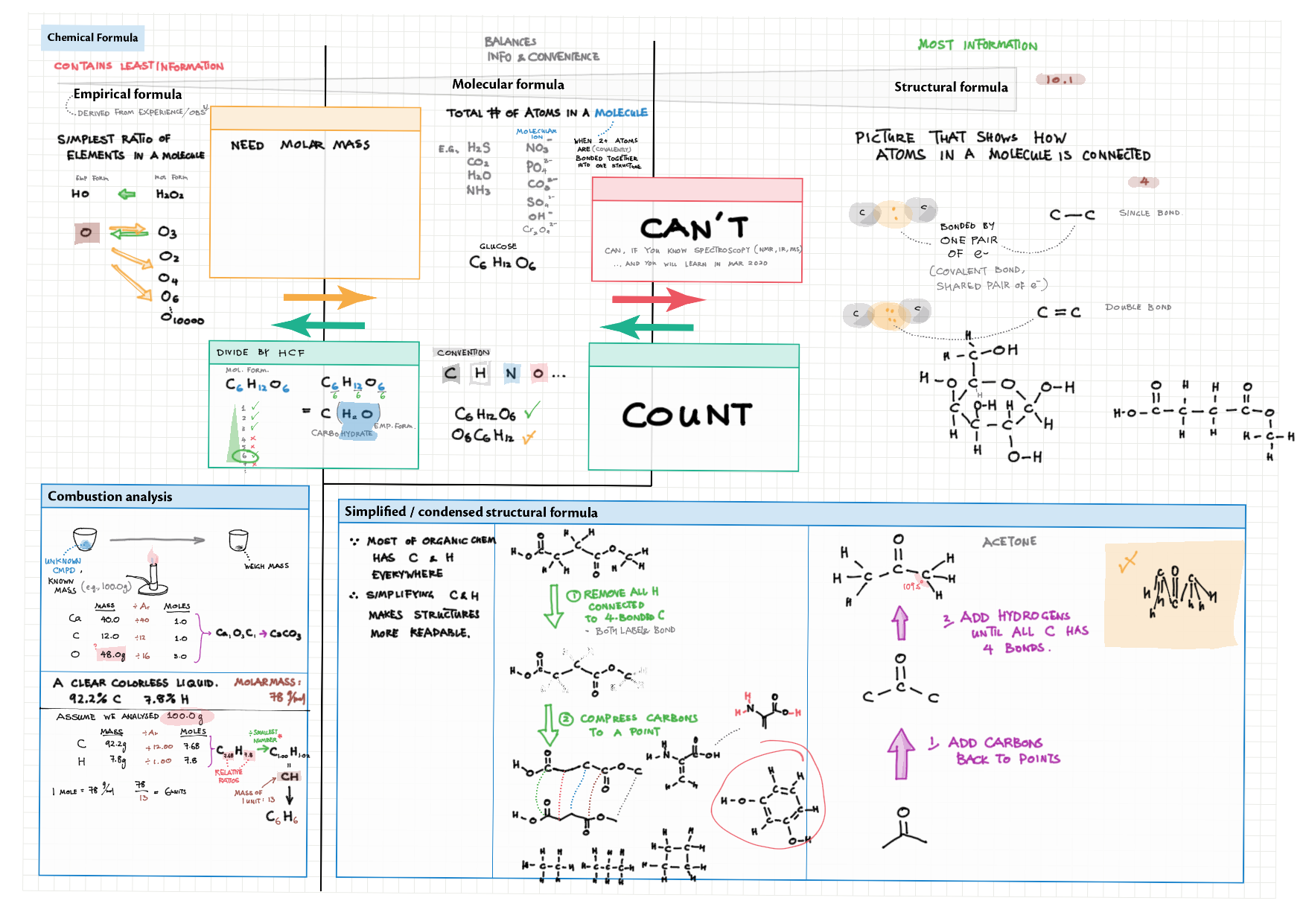
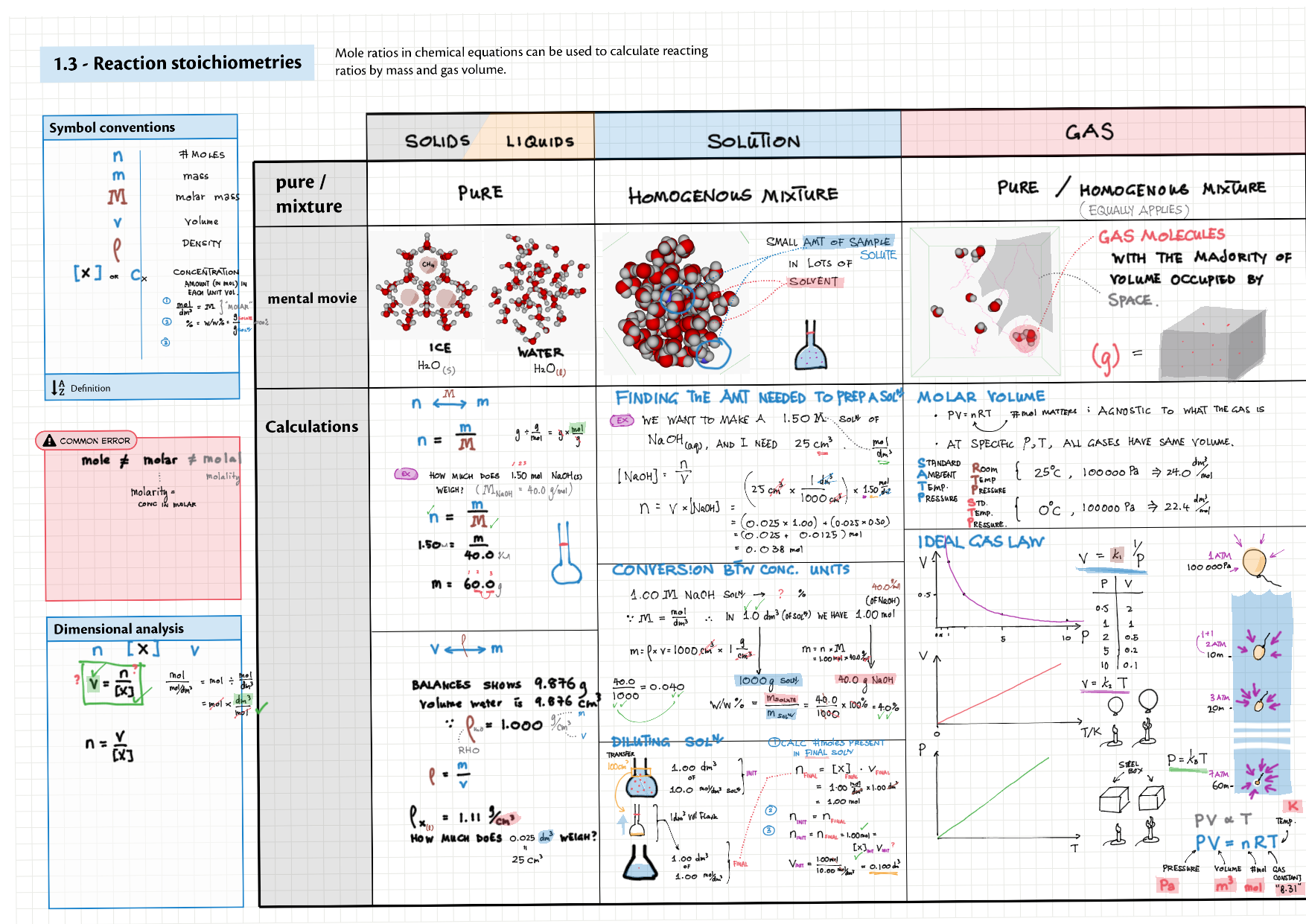
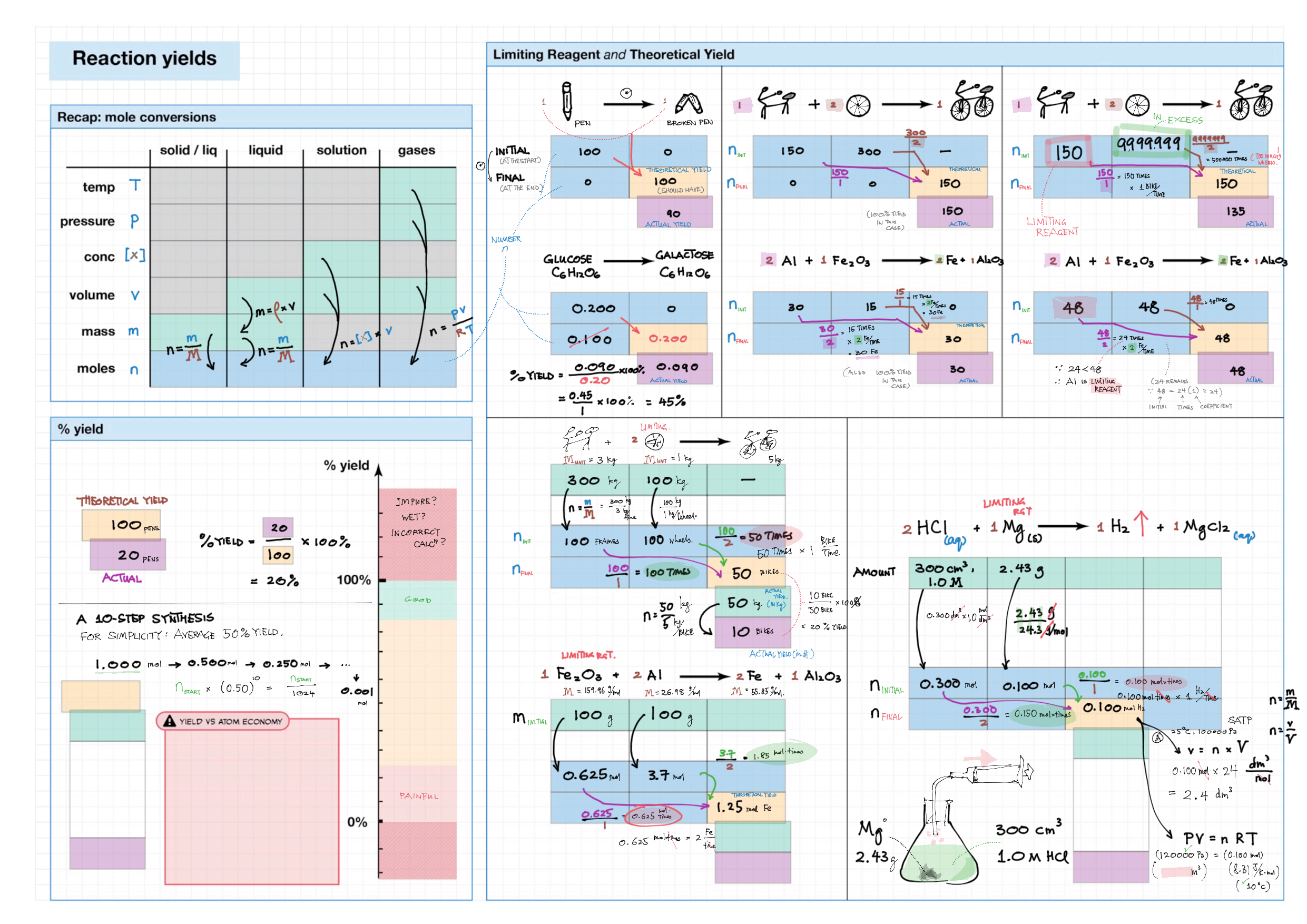
1 - Stoichiometry topics note 1, 2 Terminologies, notation, states (e.g., $\ce{^M_ZX^{p+}_n\gas{}}$ ) 1 Moles & mass calculations 1 / 10 Chemical formulas (incl. structural) 1 Chemical equations 1 Reaction stoichiometries 1 Yields
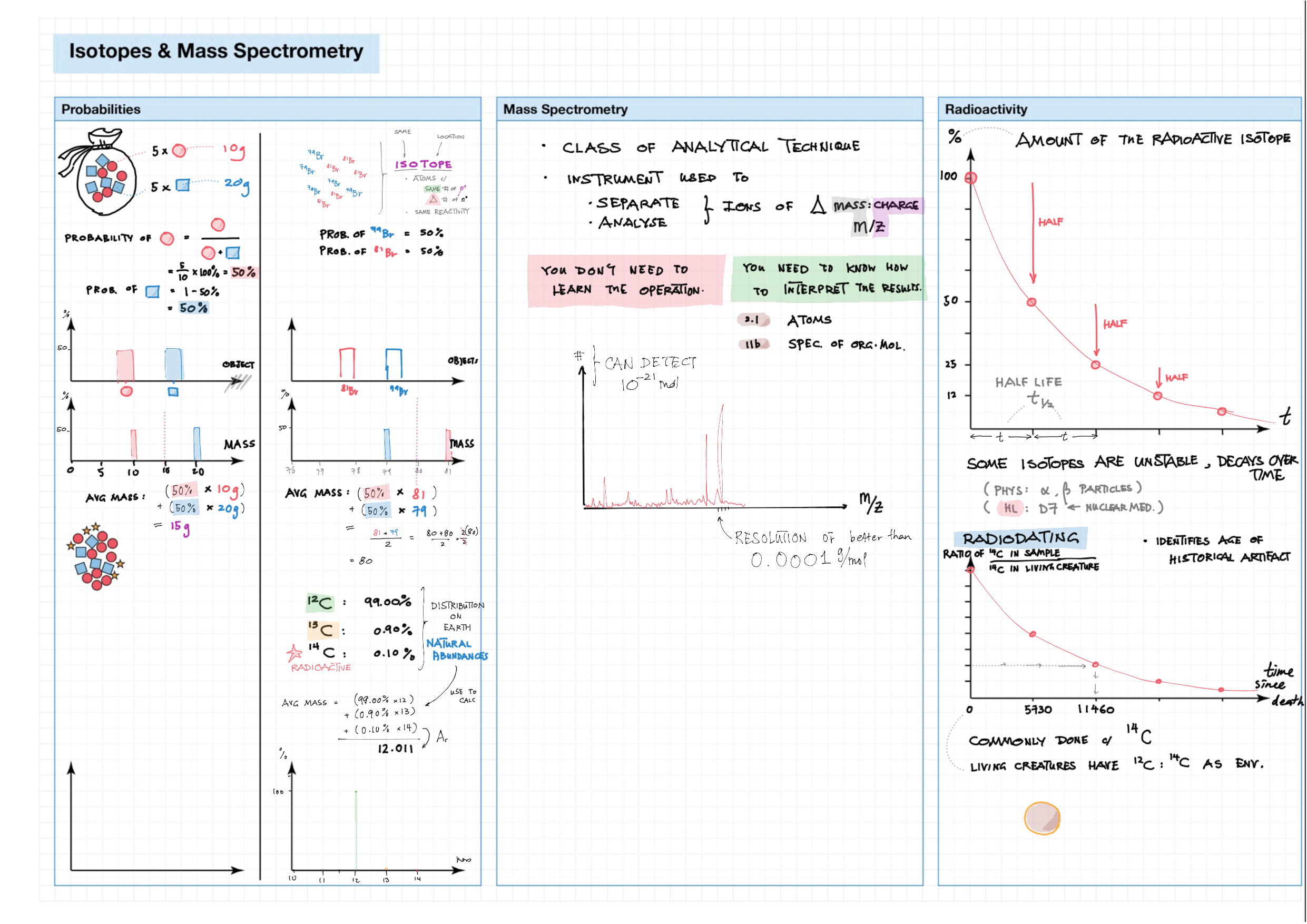
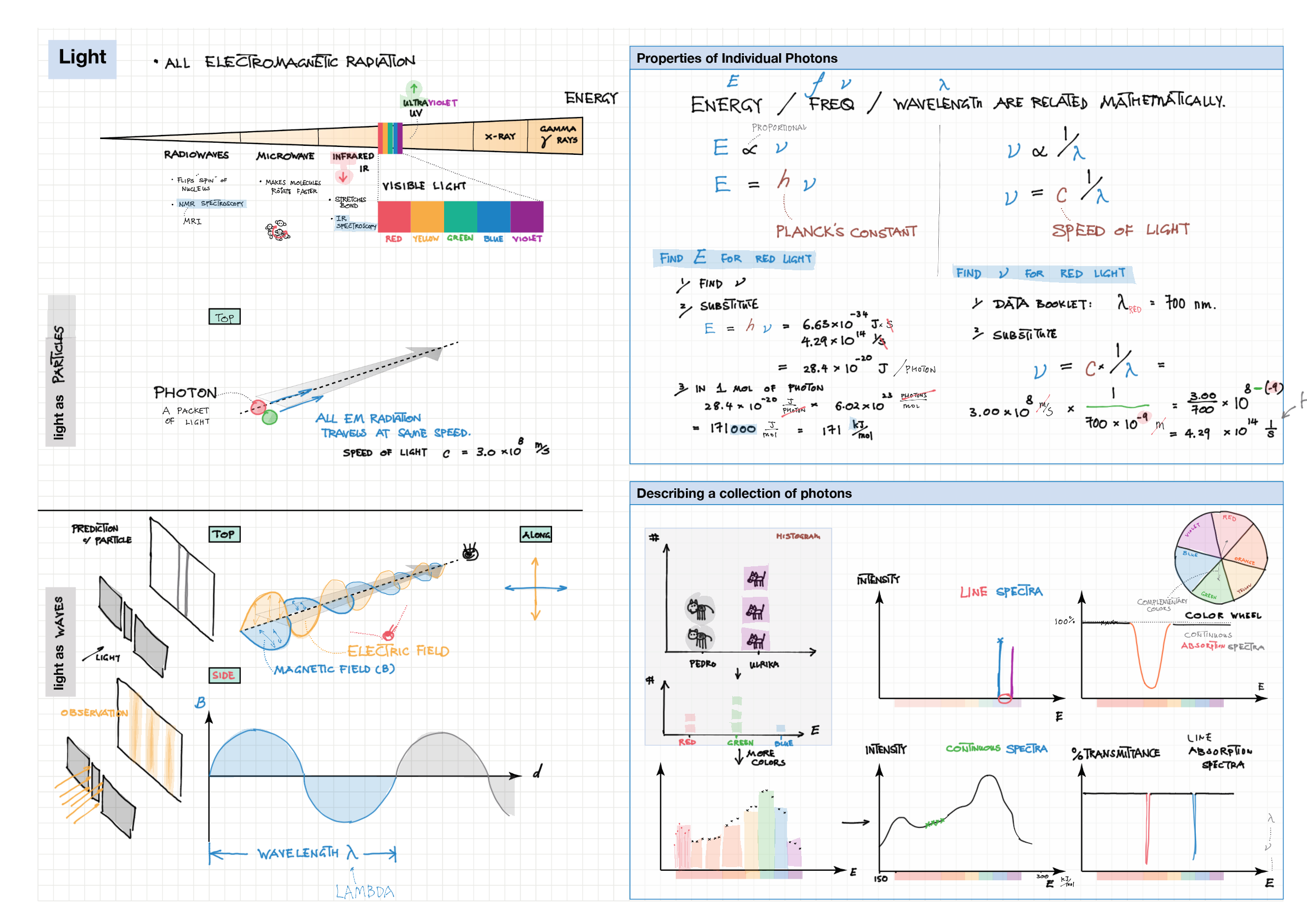
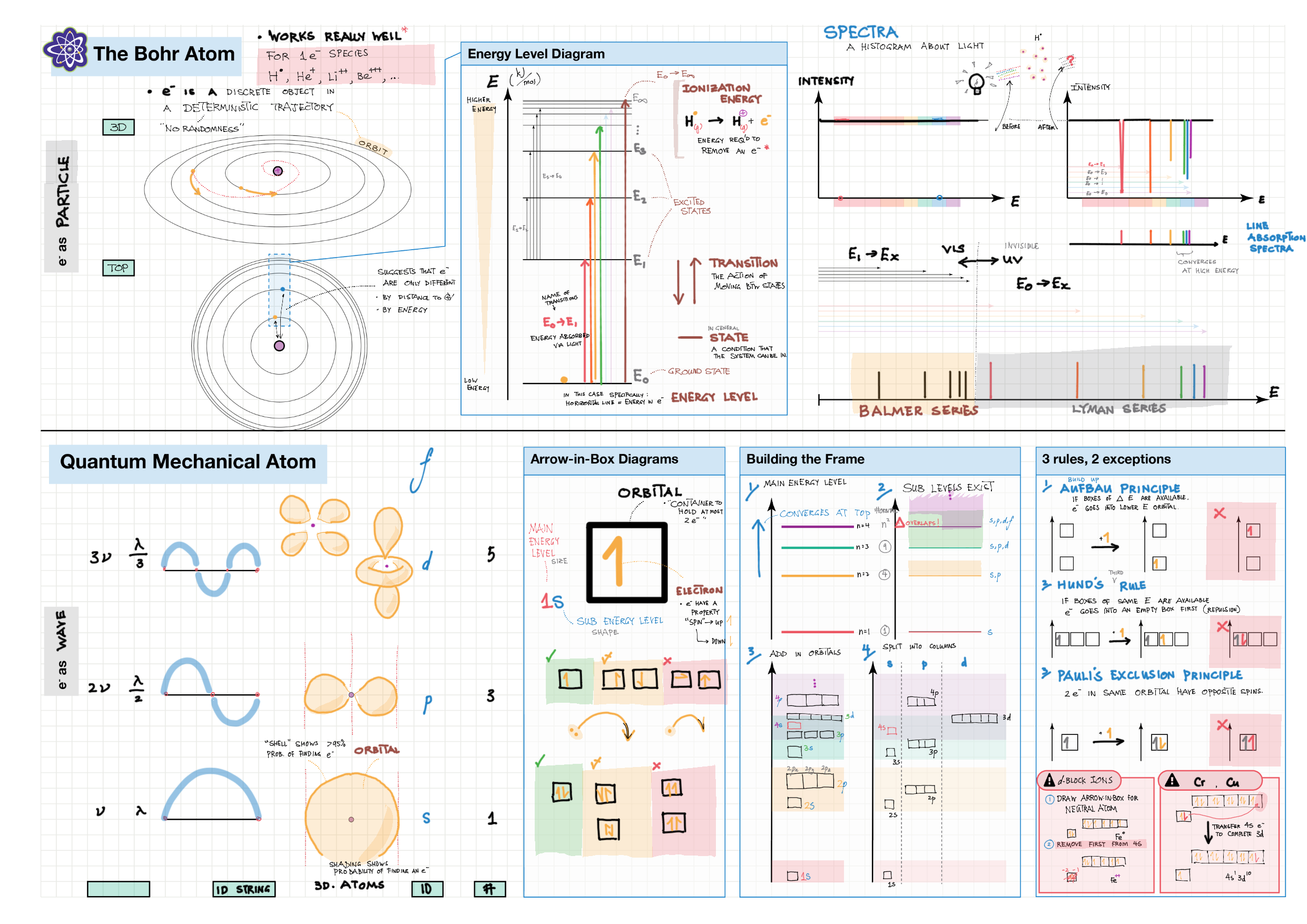
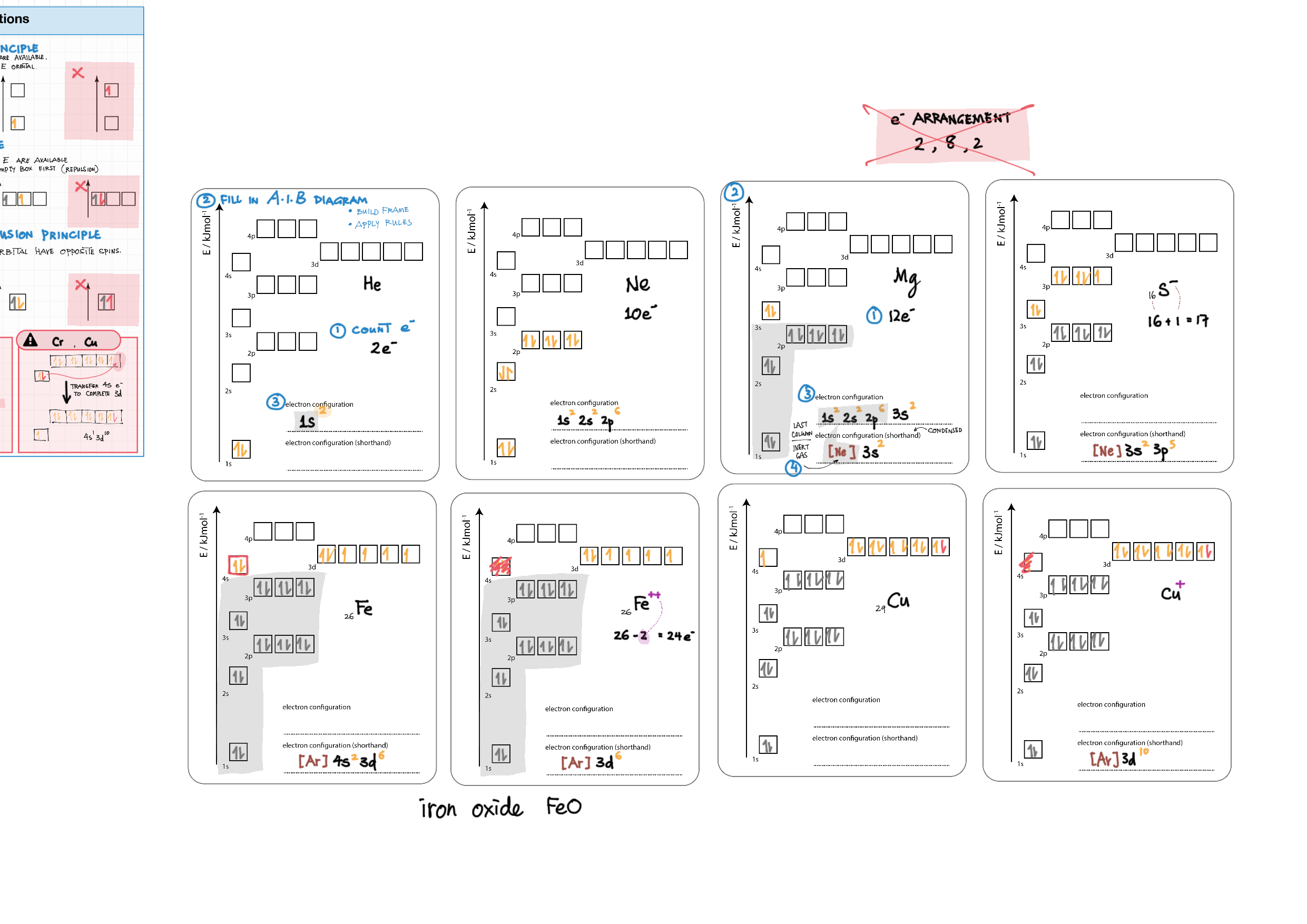
2/12 - Atomic structure topics note 2 Isotopes, Mass Spec, Radioactivity 2/12 Light and Spectra 2 Bohr/QM atom, Energy Level Diagram, Spectra, e- config 2 e- config examples
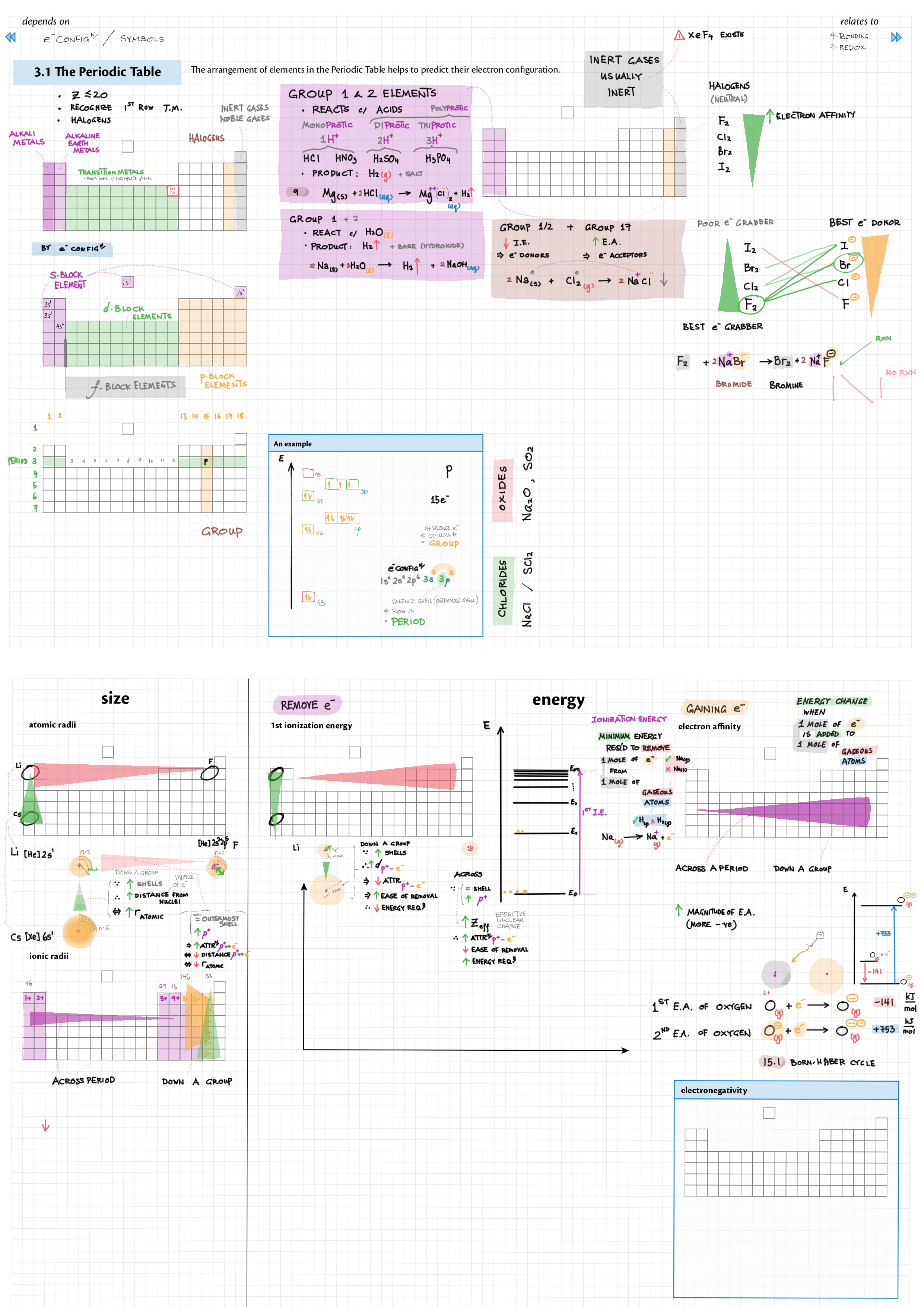
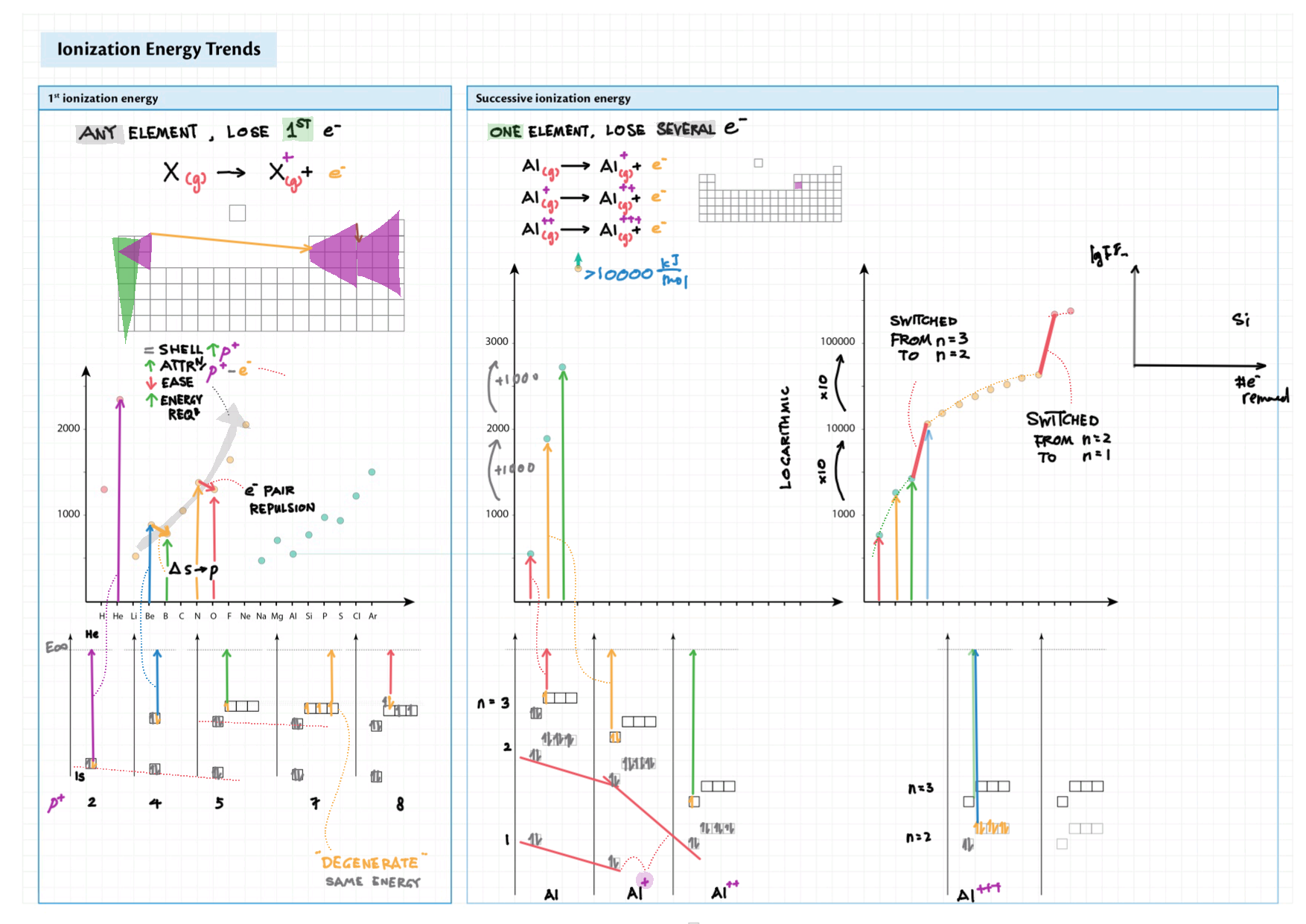
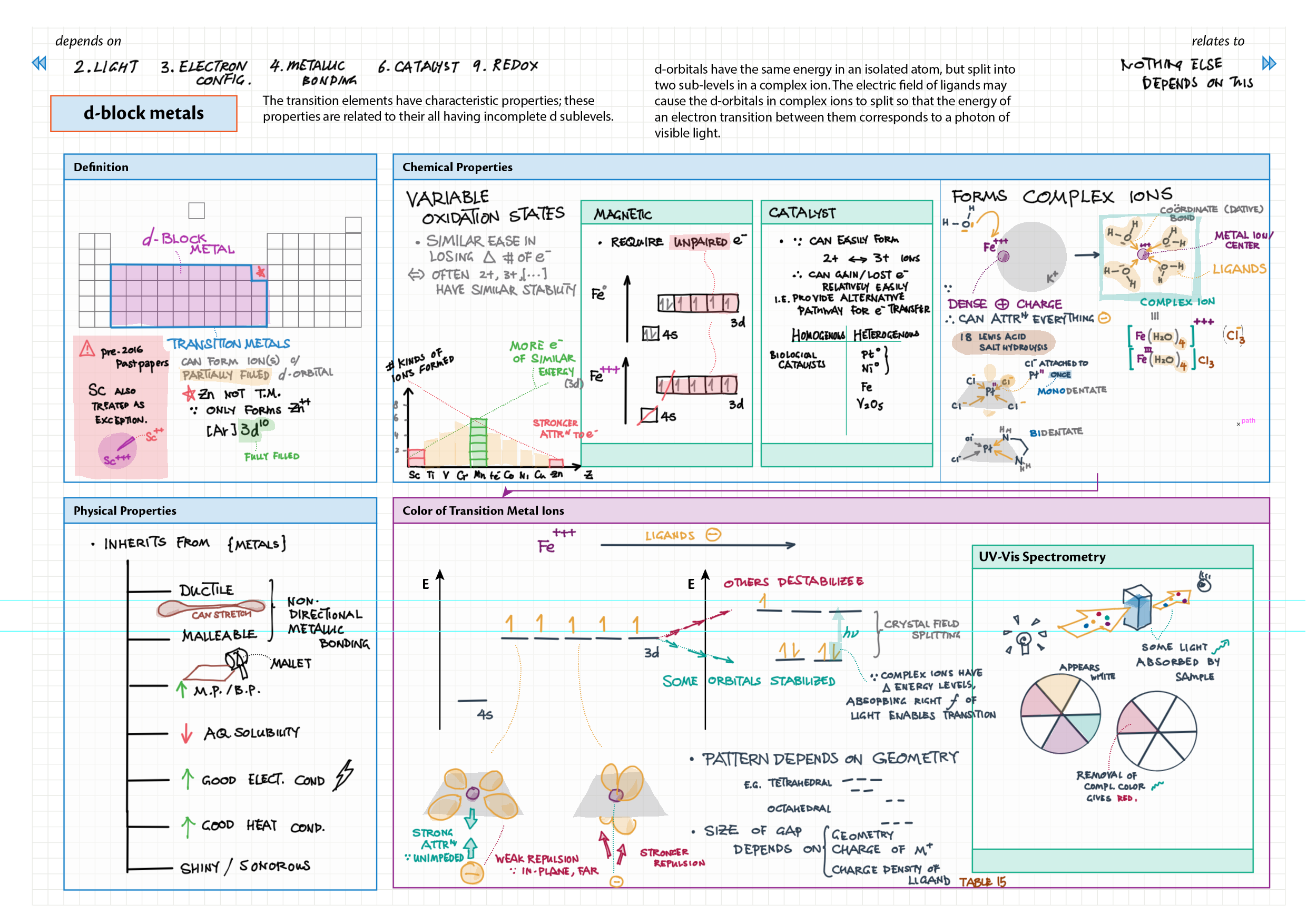
3/13 - Periodicity topics note 3 Terminology (block, groups, periods), periodic trends (all 5) 3 In-depth ionization energy 13 d-block metals
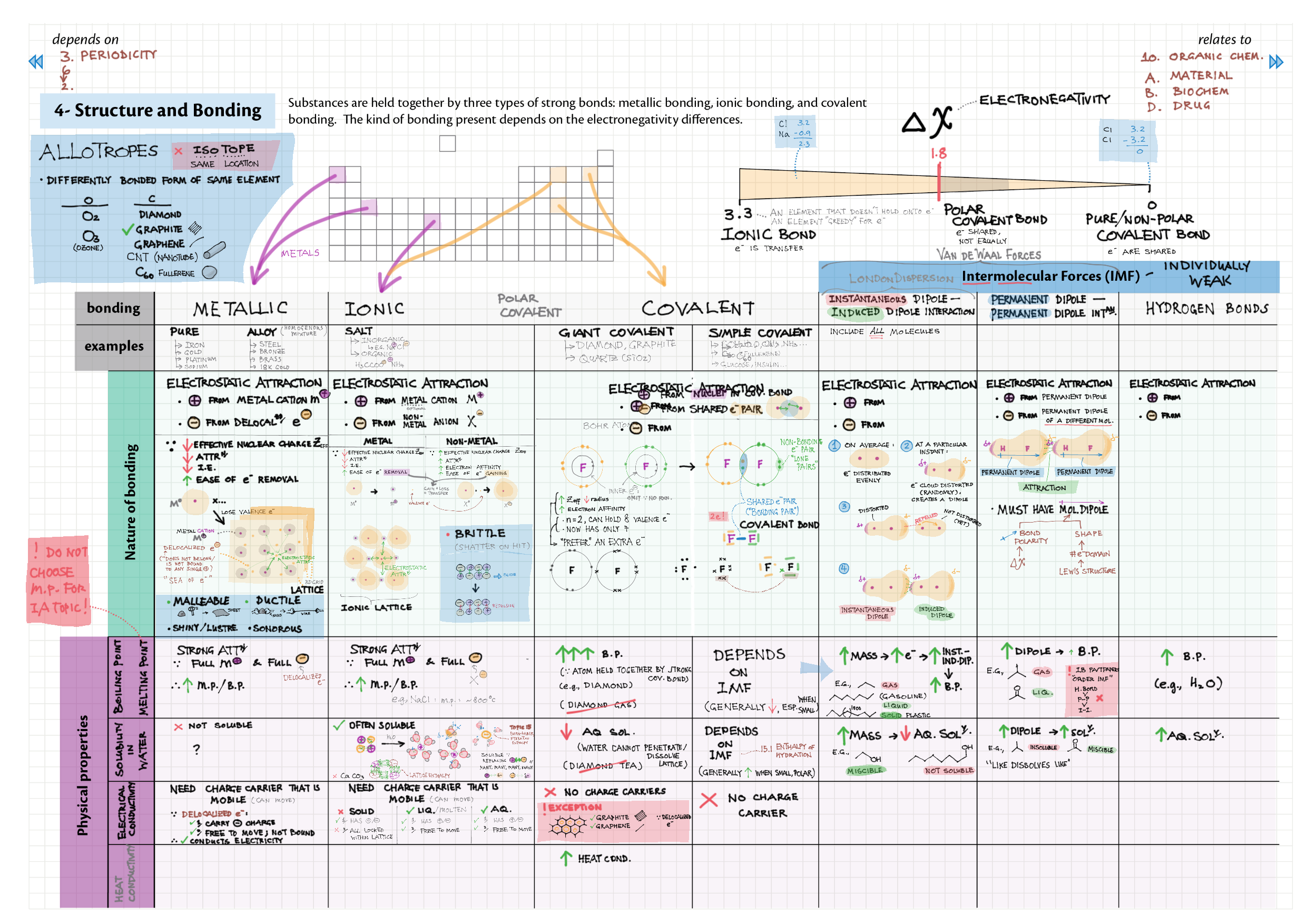
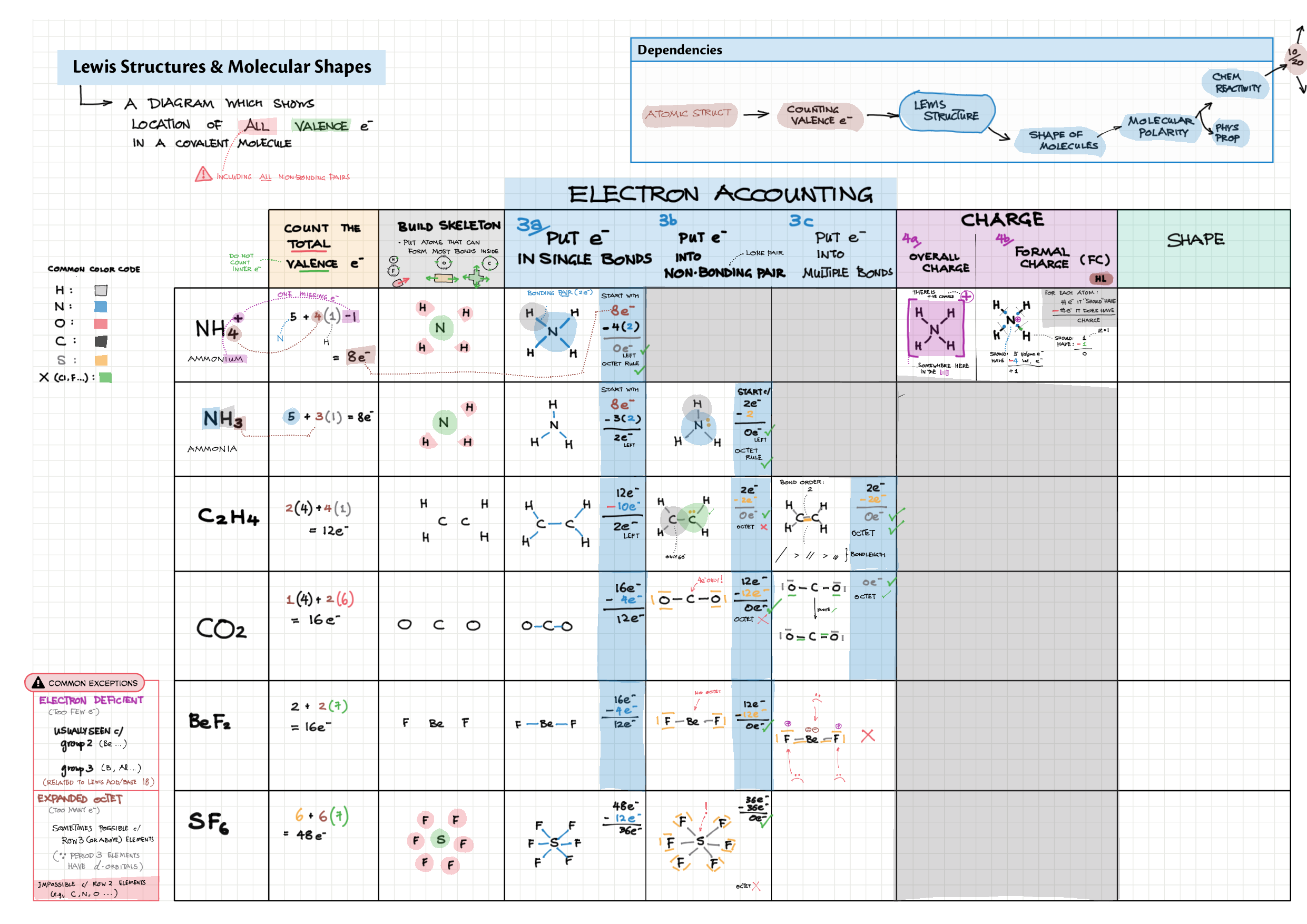
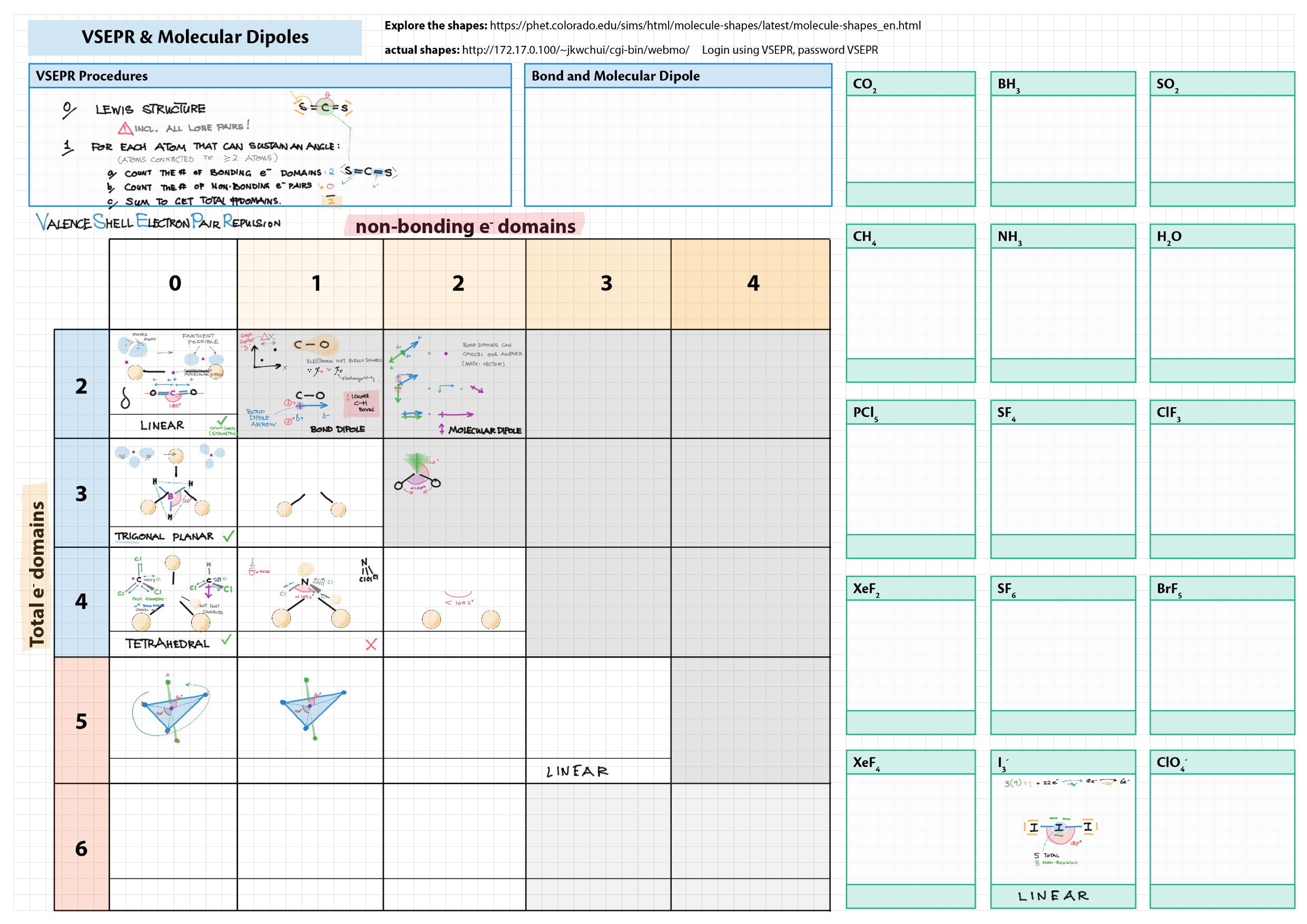
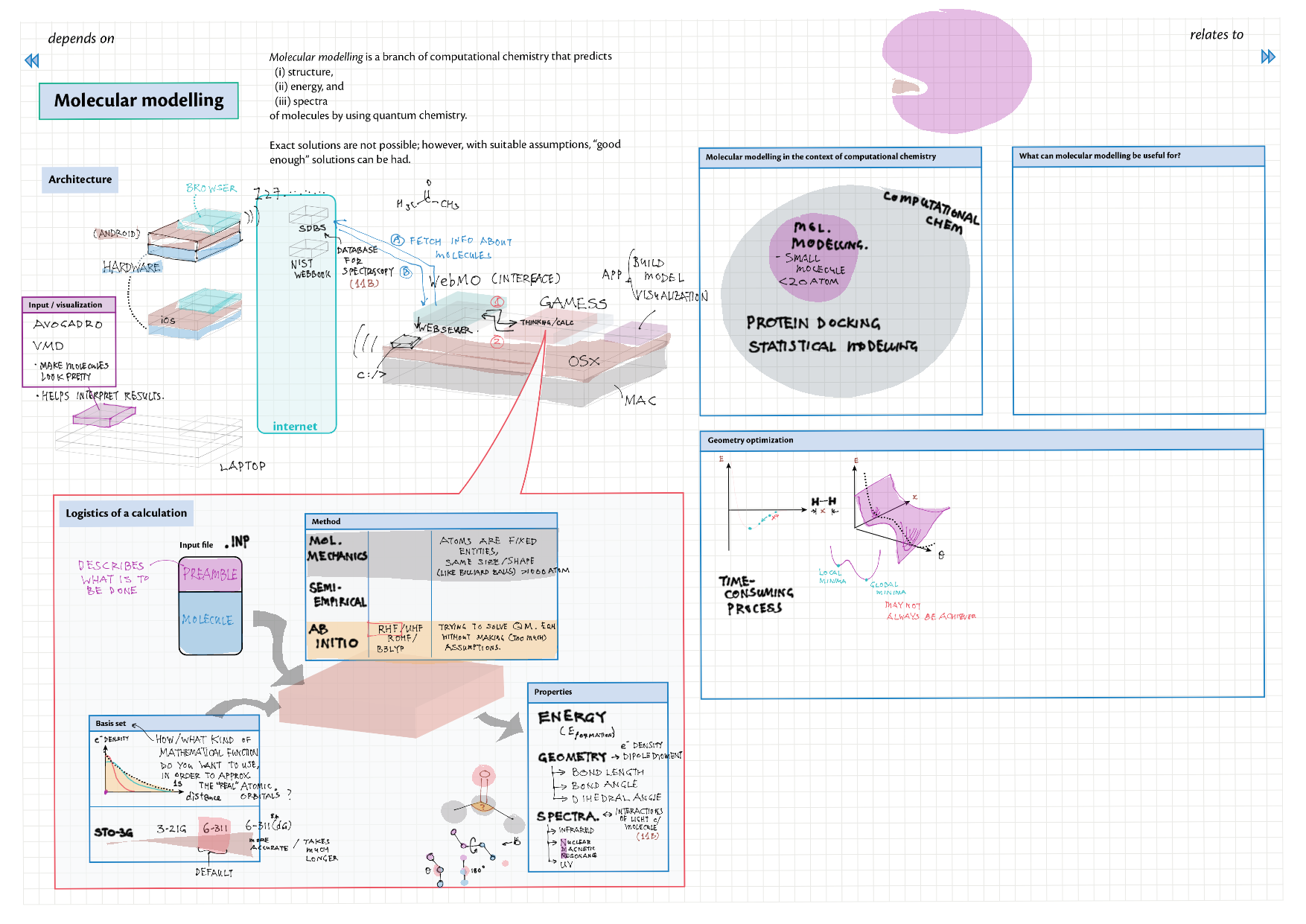
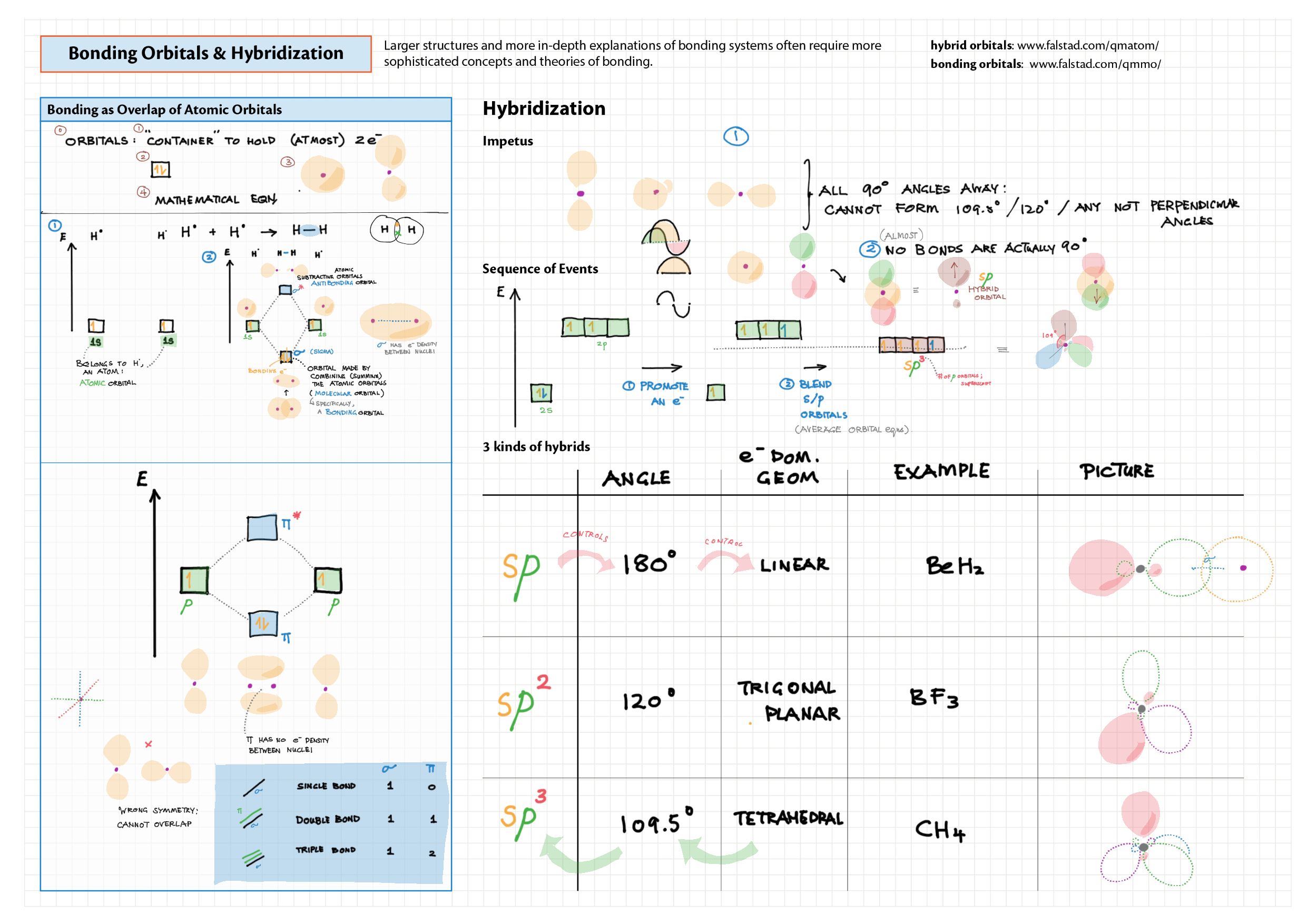
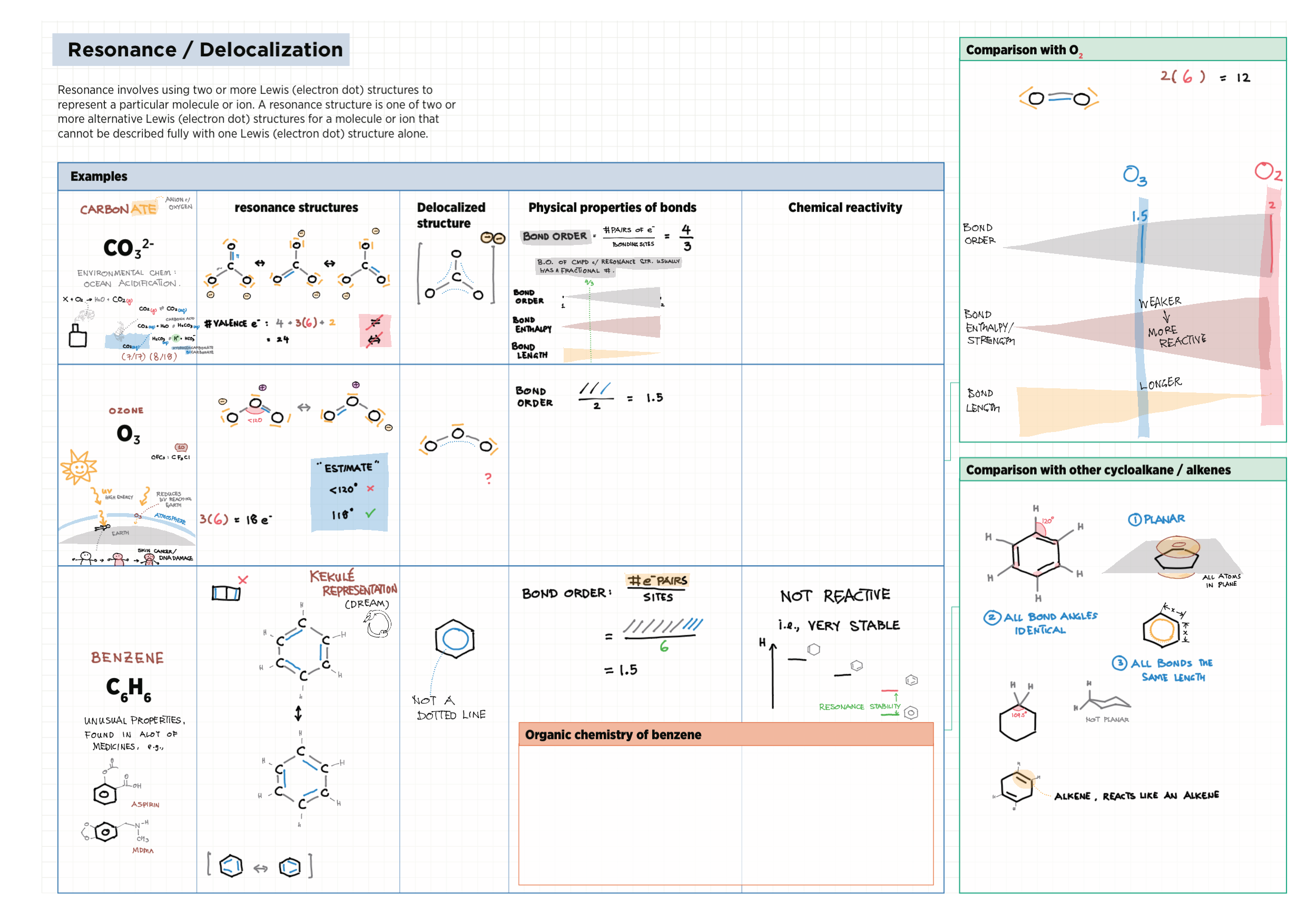
4/14 - Structure & Bonding topics note 4 Structure Bonding overview & comparison 4 Lewis structures 4 VSEPR & molecular dipole 4 Molecular modelling 14 Bonding orbitals and Hybridization 14 Resonance and Delocalization
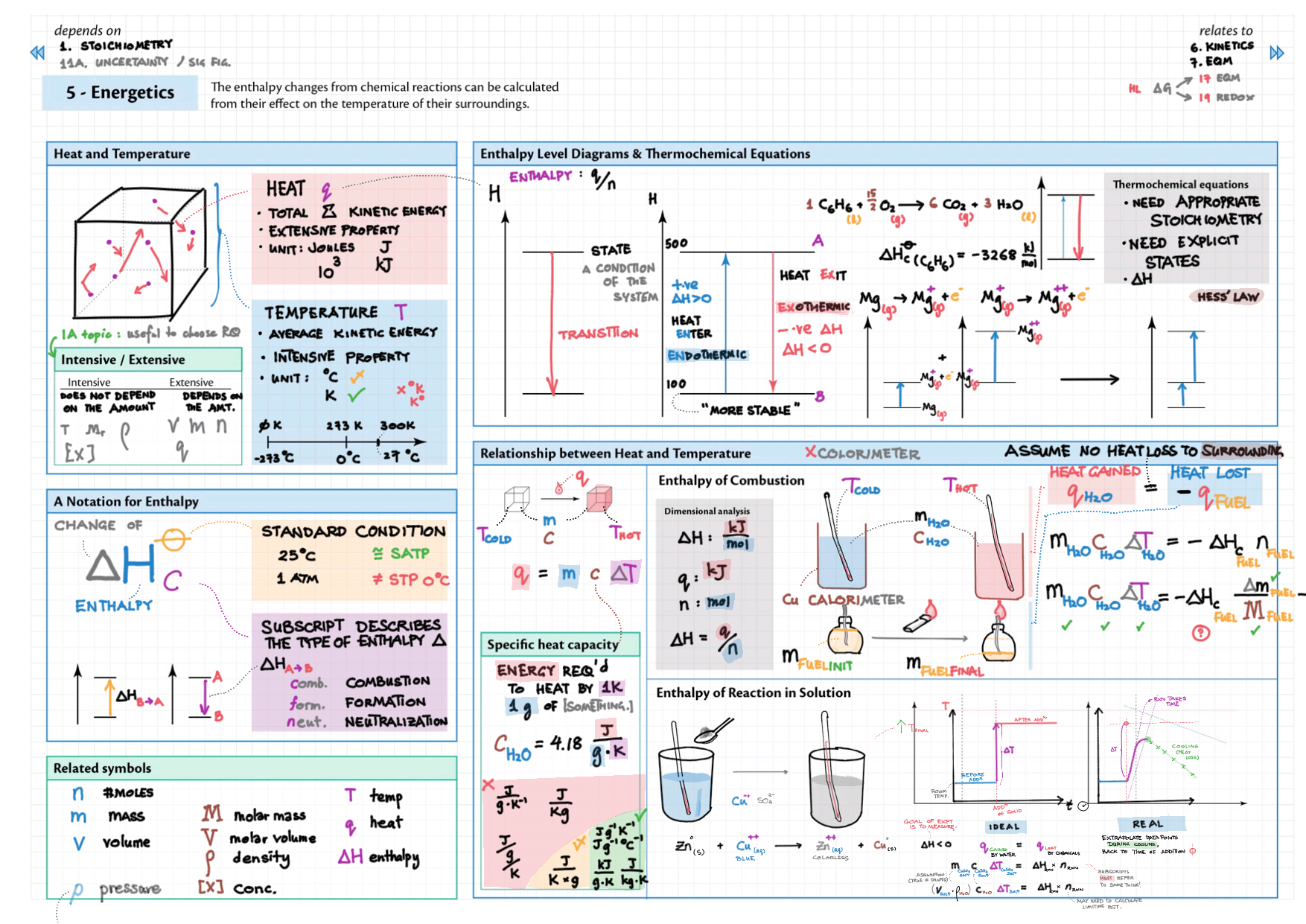
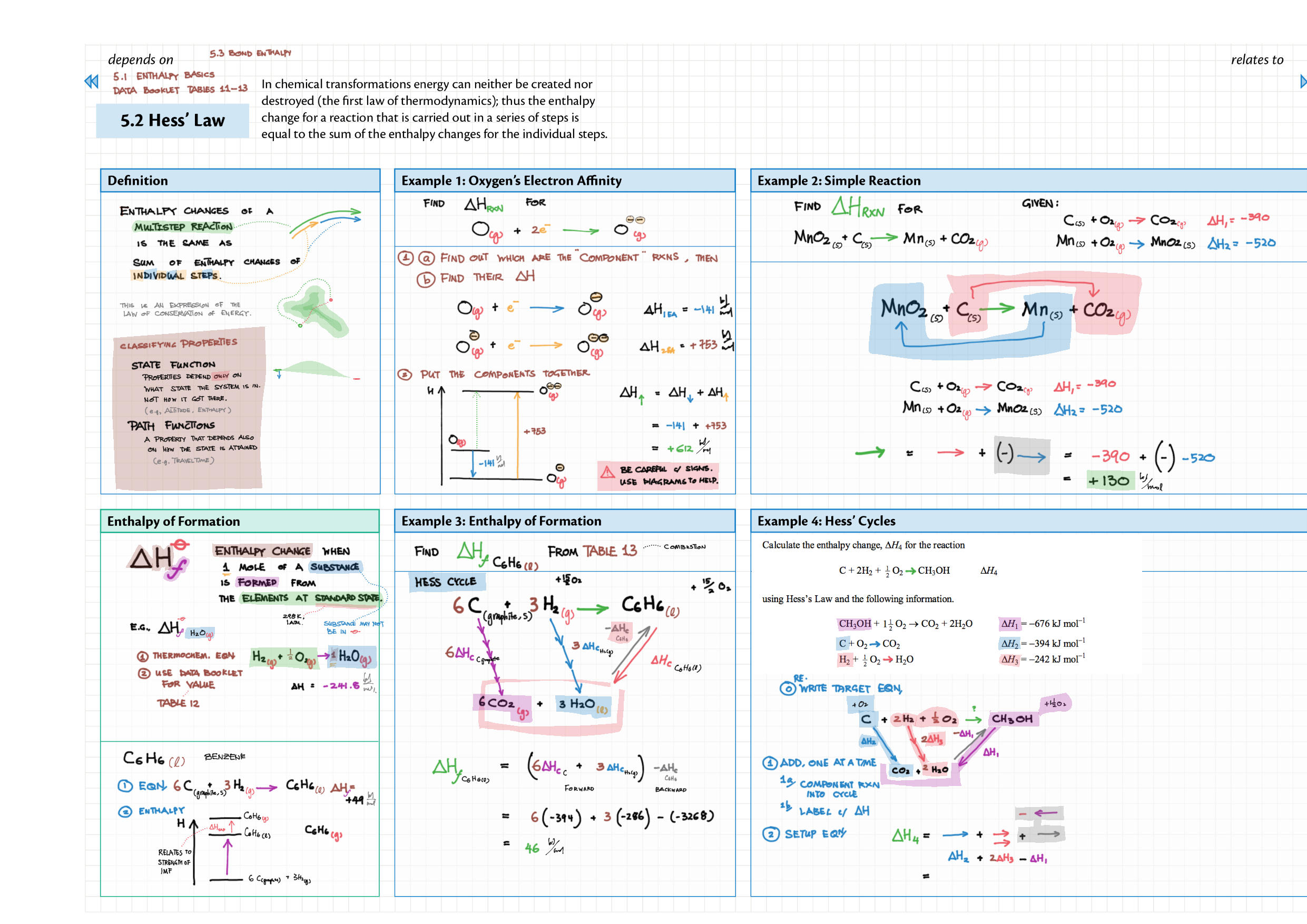
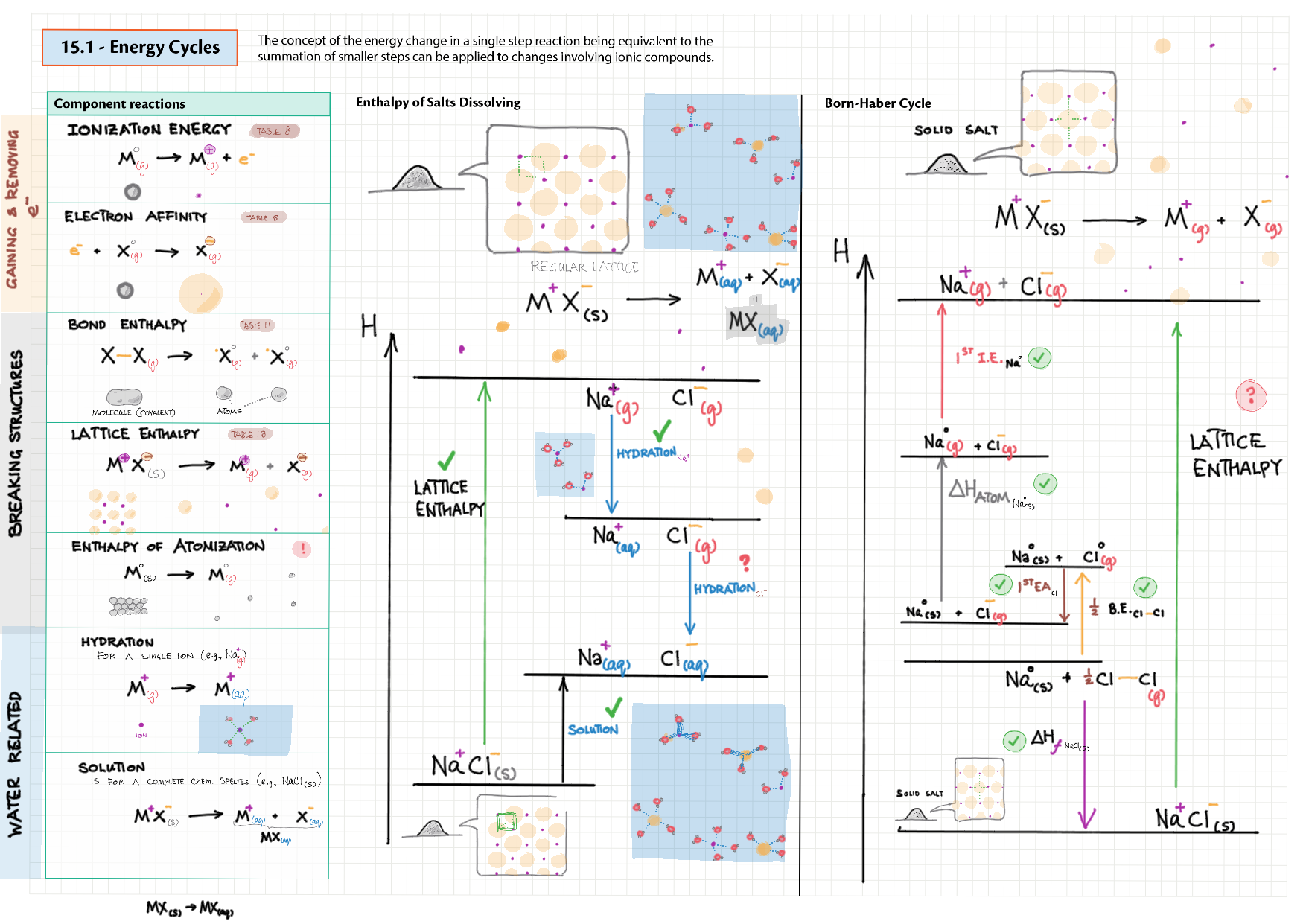
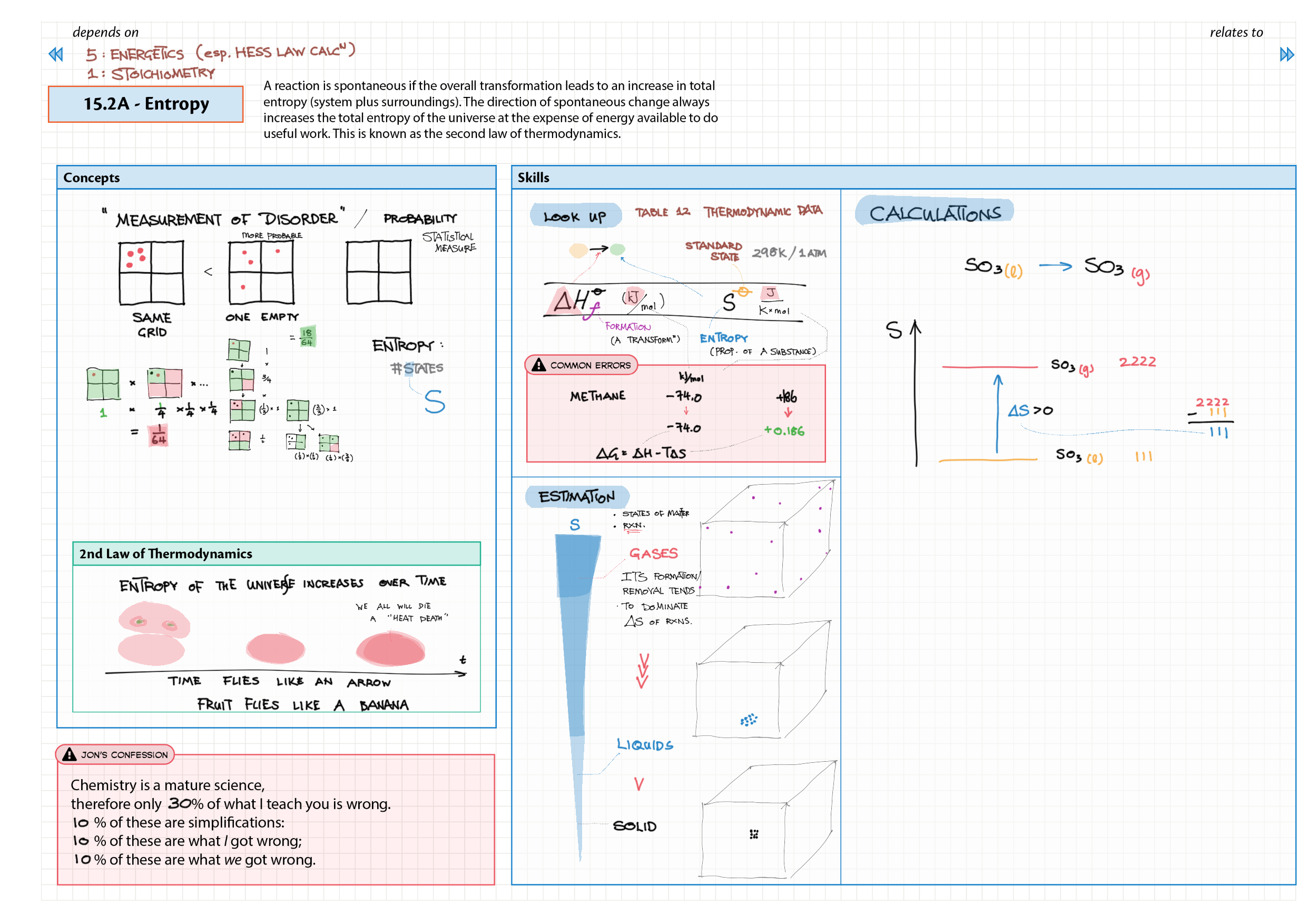
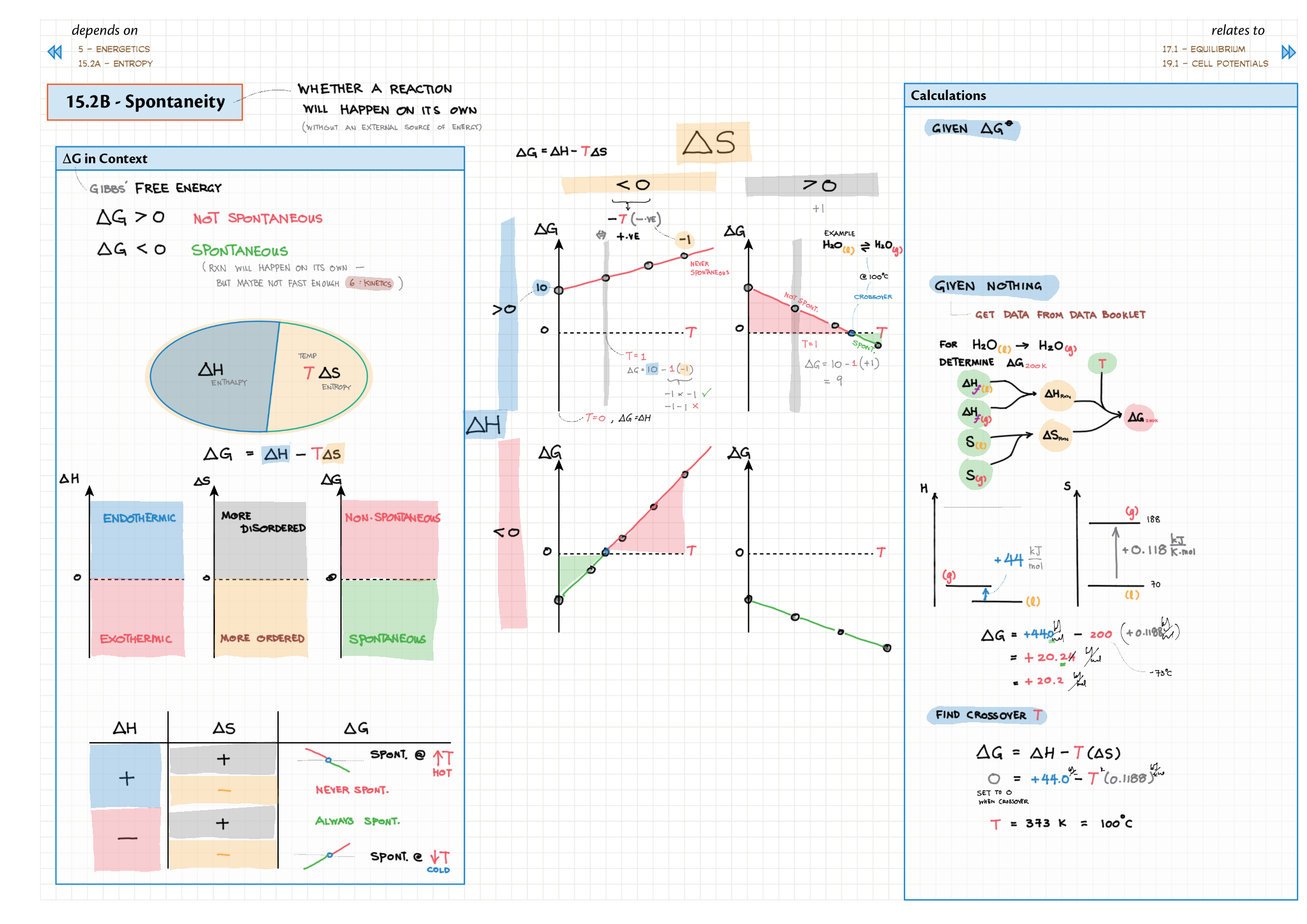
5/15 - Energetics topics note 5 Enthalpy - notation, diagram; heat changes, experiments 5 Hess' Law 5 Bond enthalpy 15 Energy cycles 15 Entropy 15 Spontaneity
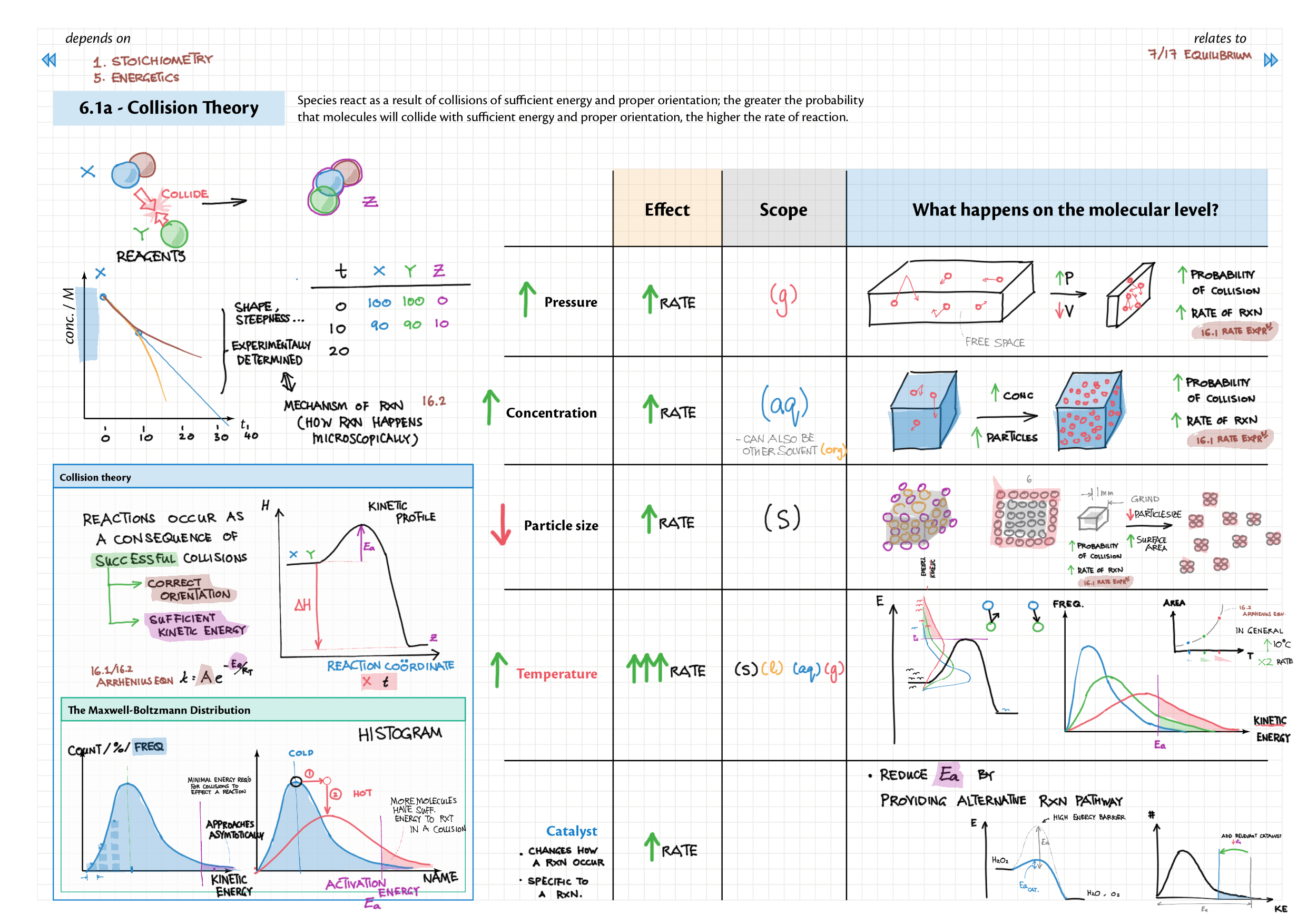
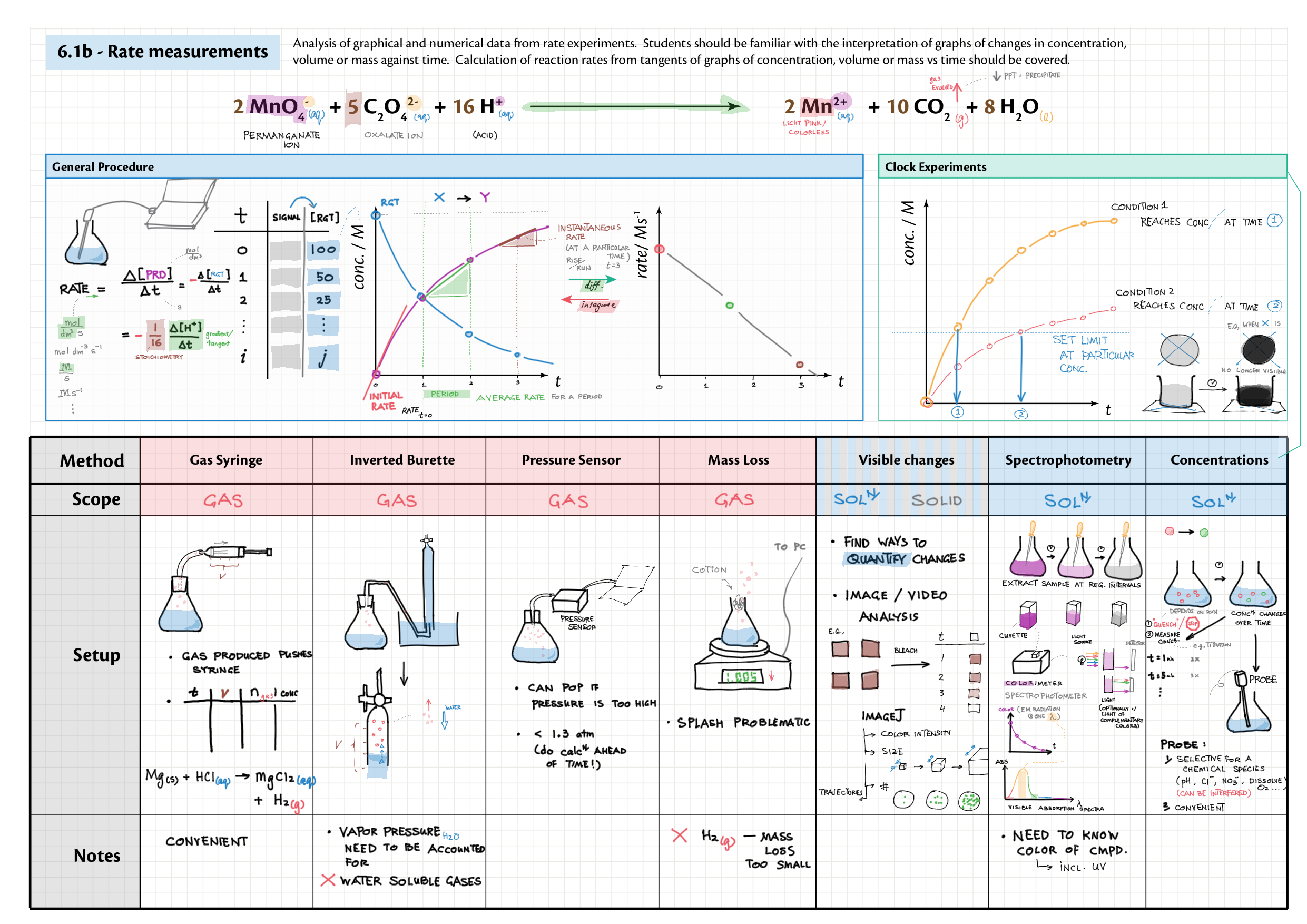
6/16 - Kinetics topics note 6 Collision theory 6 Rate measurements 6 Sample rate calculation 16 Rate expressions 16 Arrhenius equation / activation energy
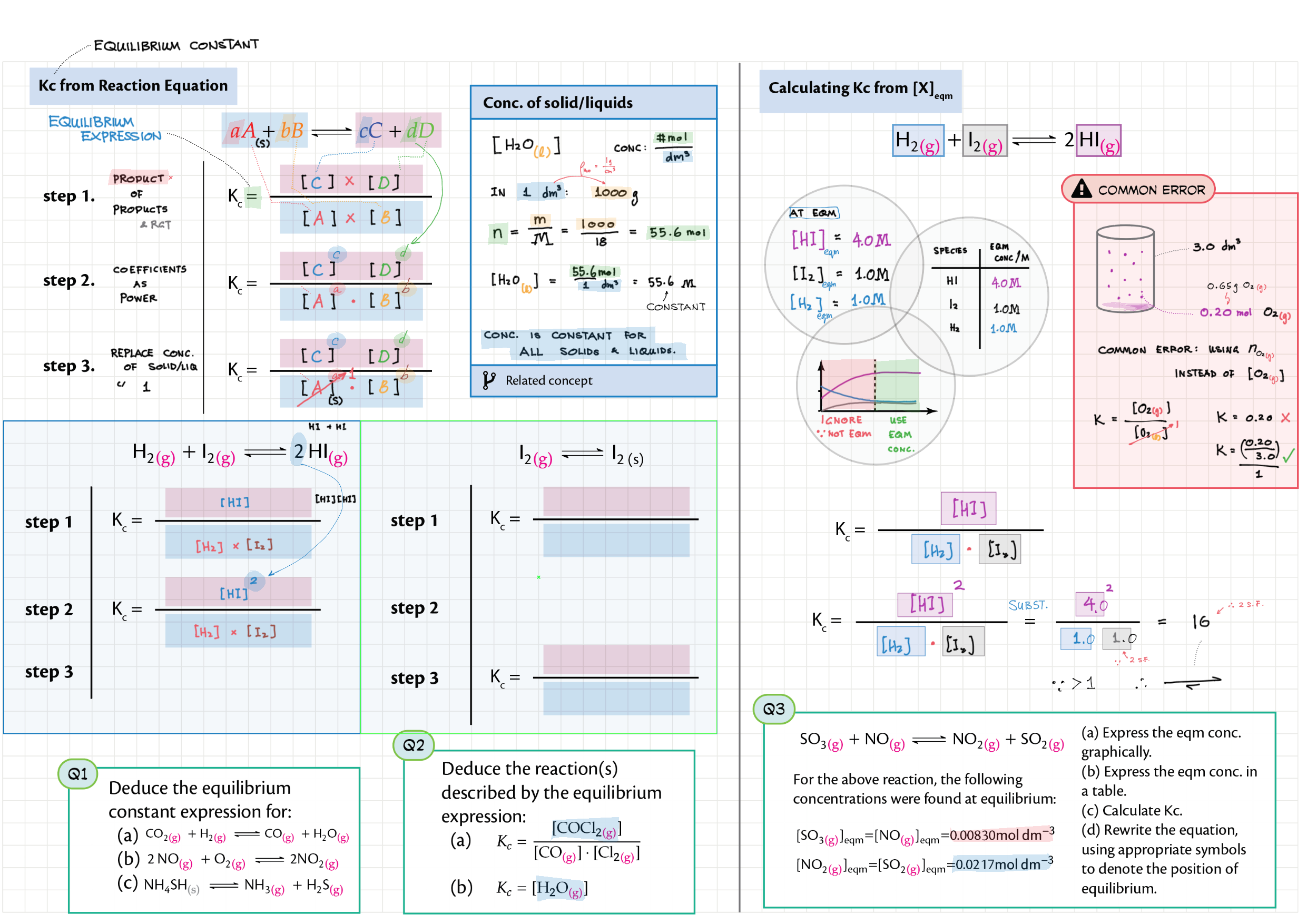
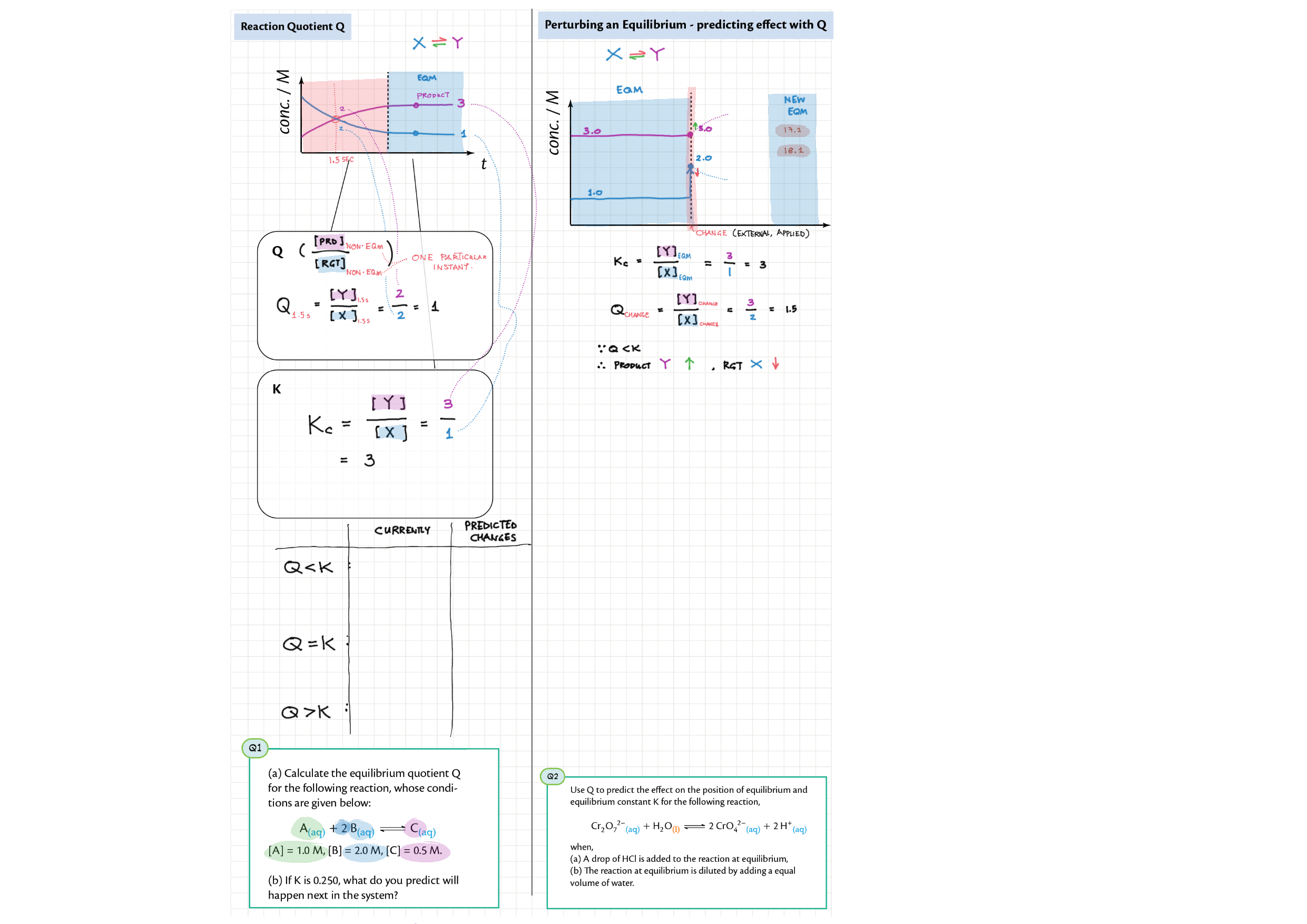
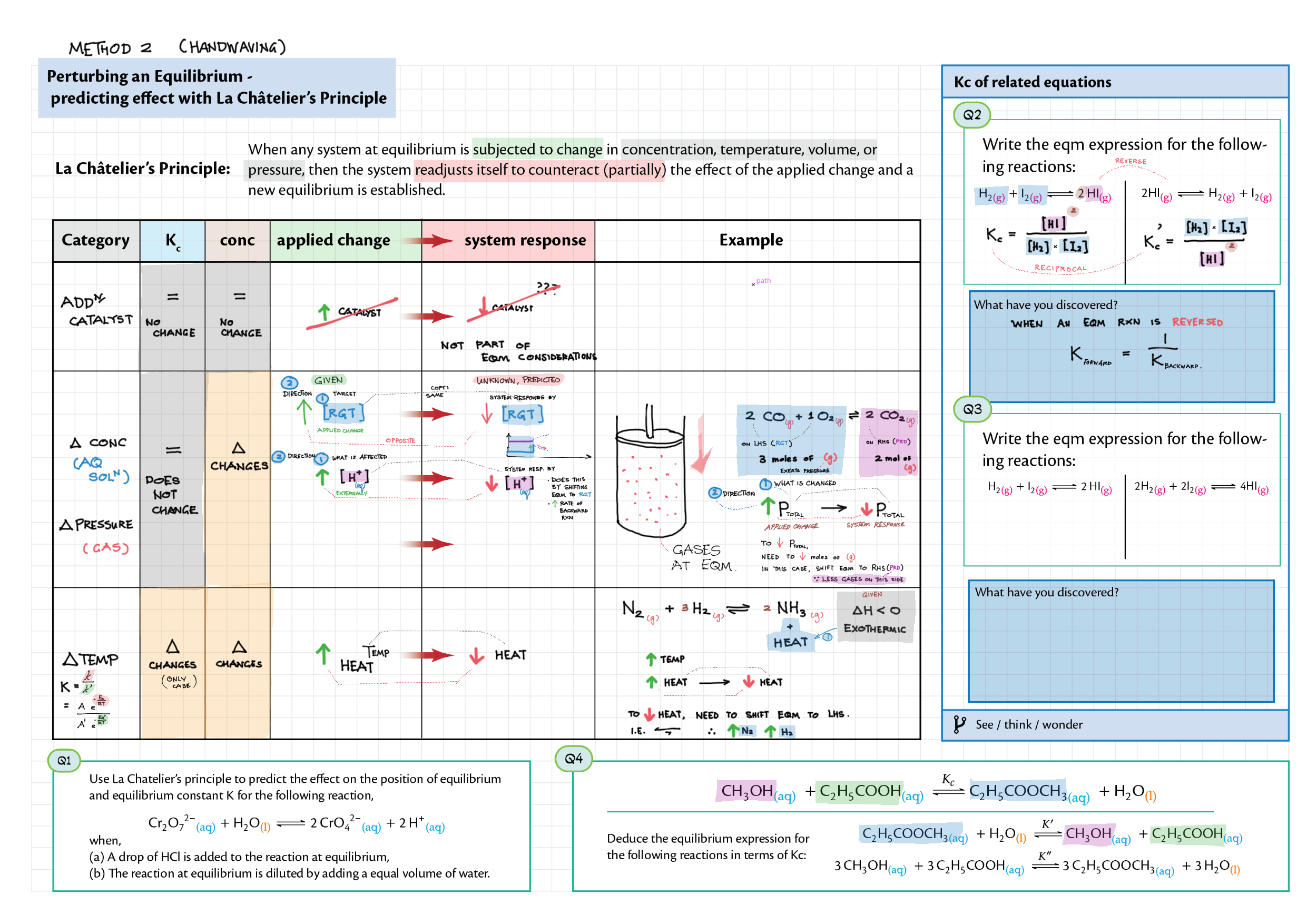
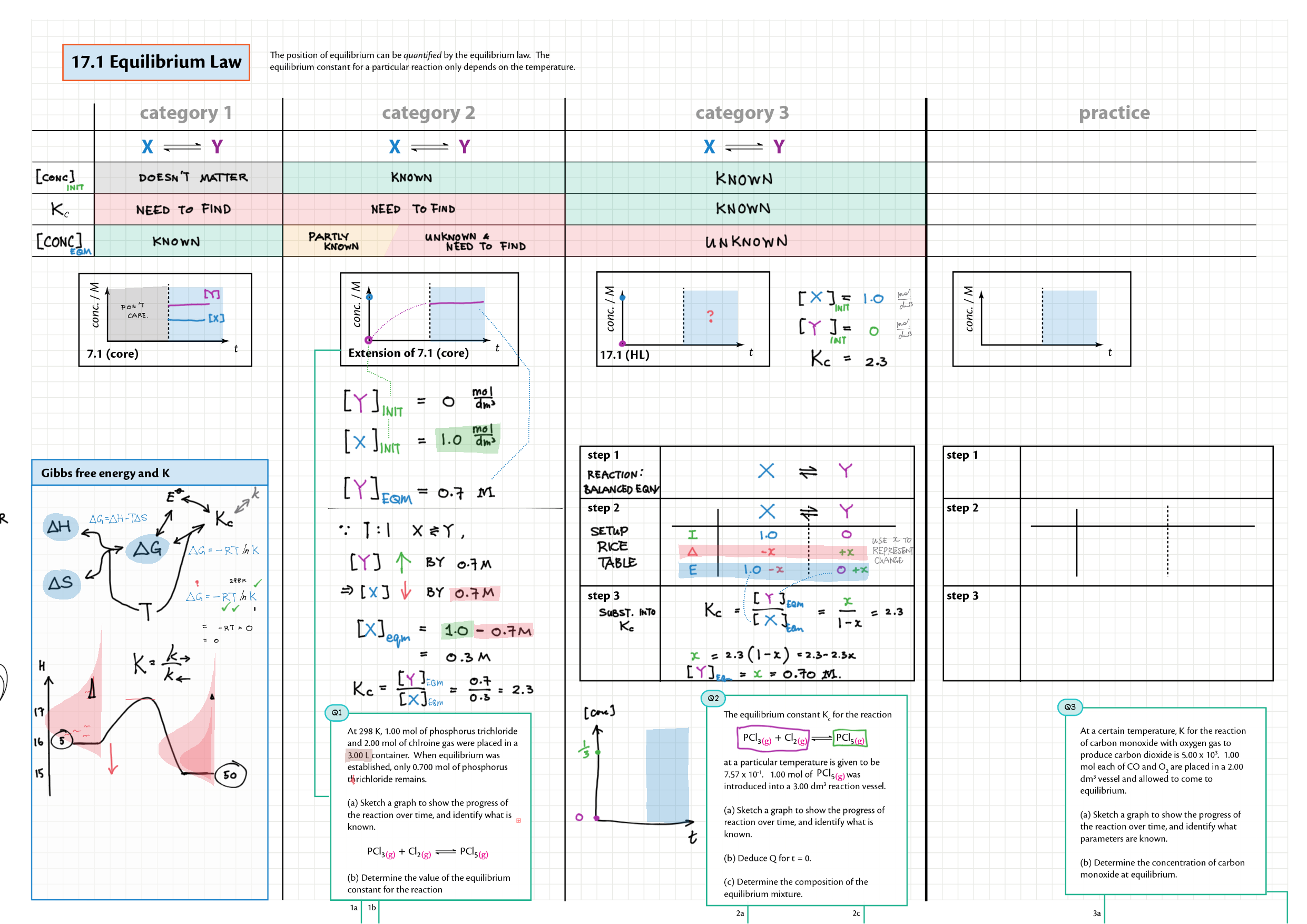
7/17 - Equilibrium topics note 7 Equilibrium intro 7 Equilibrium constant Kc 7 Reaction quotient Q 7 La Chatelier's principle, re-arranging Kc expressions 17 Equilibrium law / calc
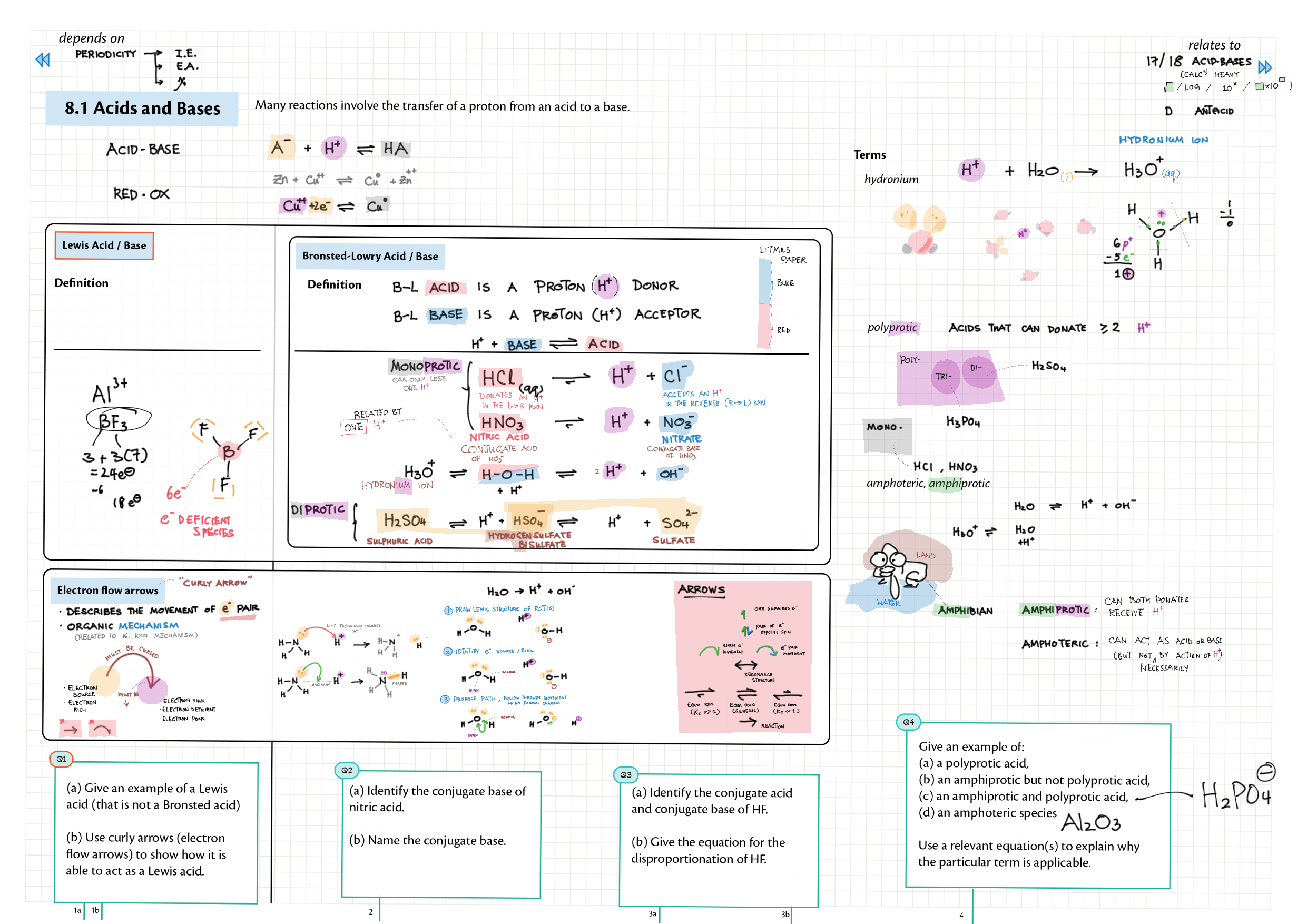
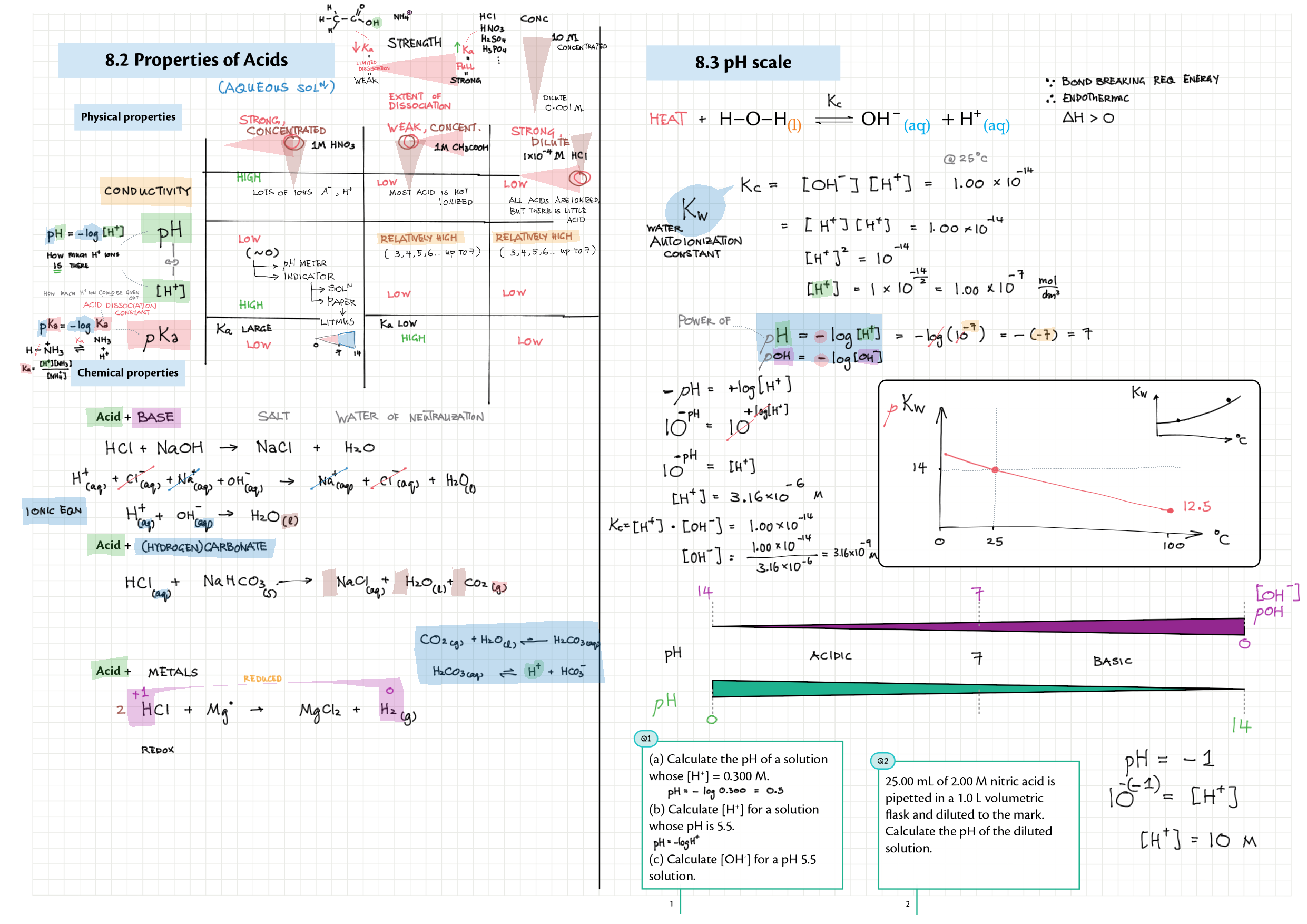
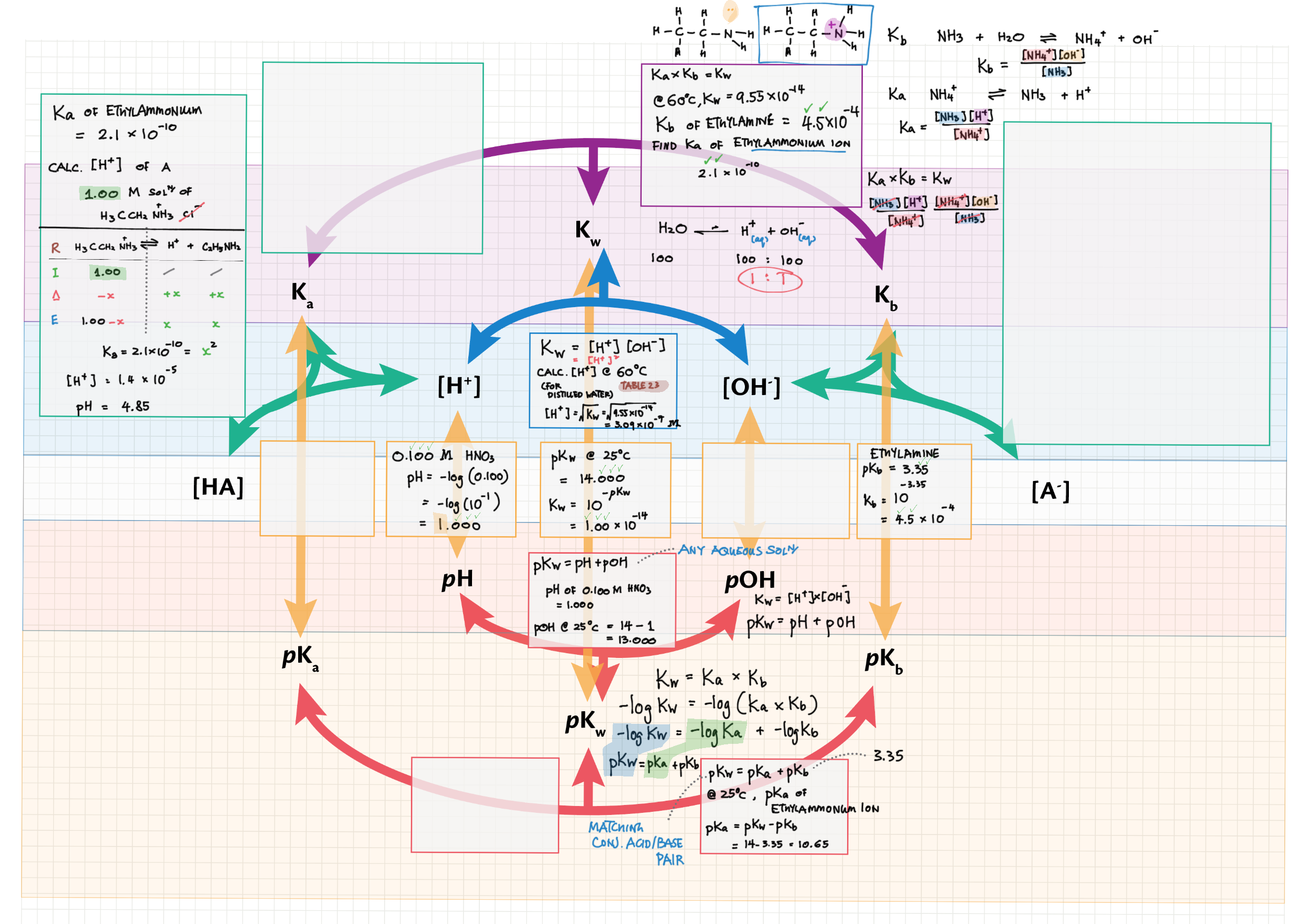
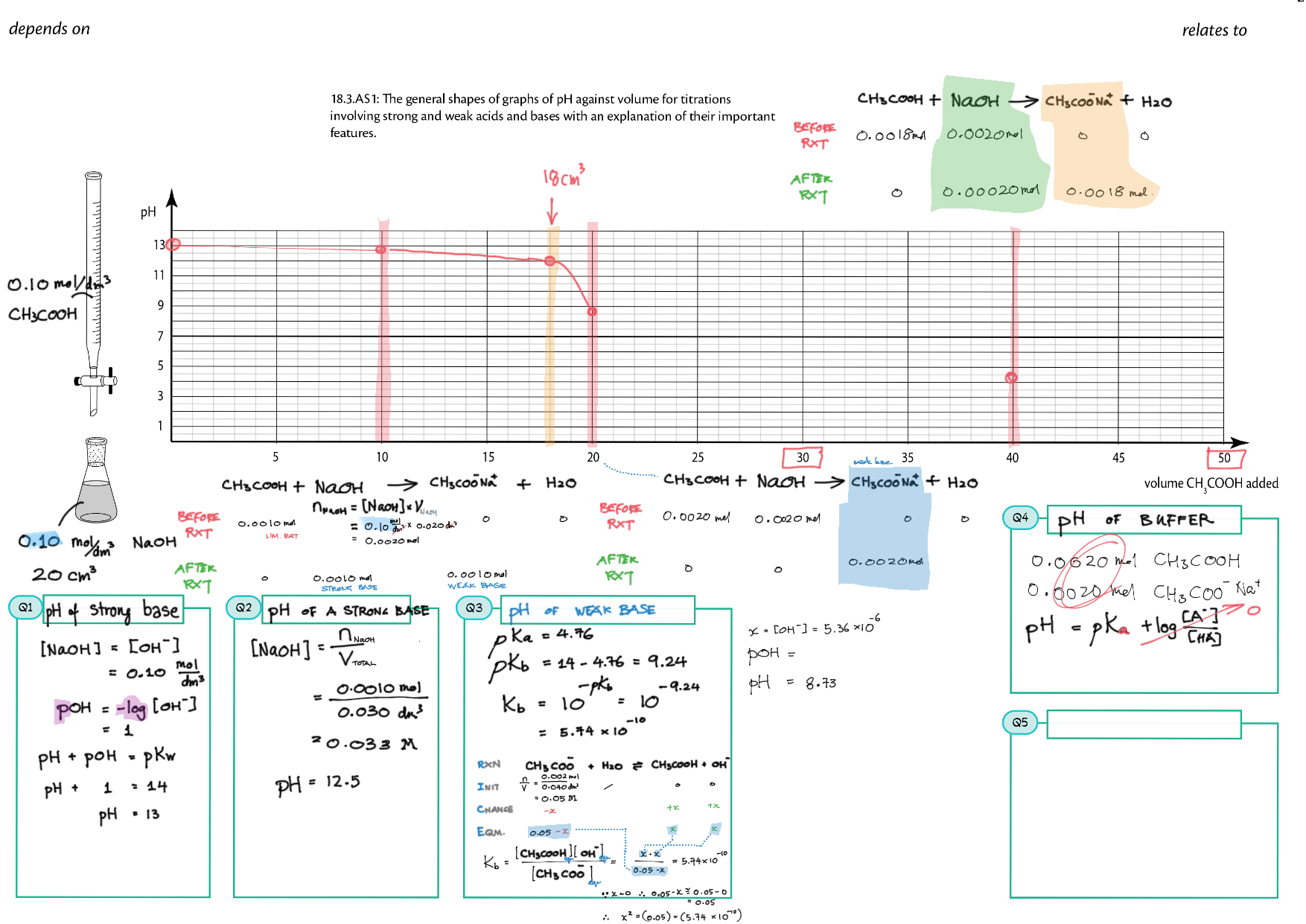
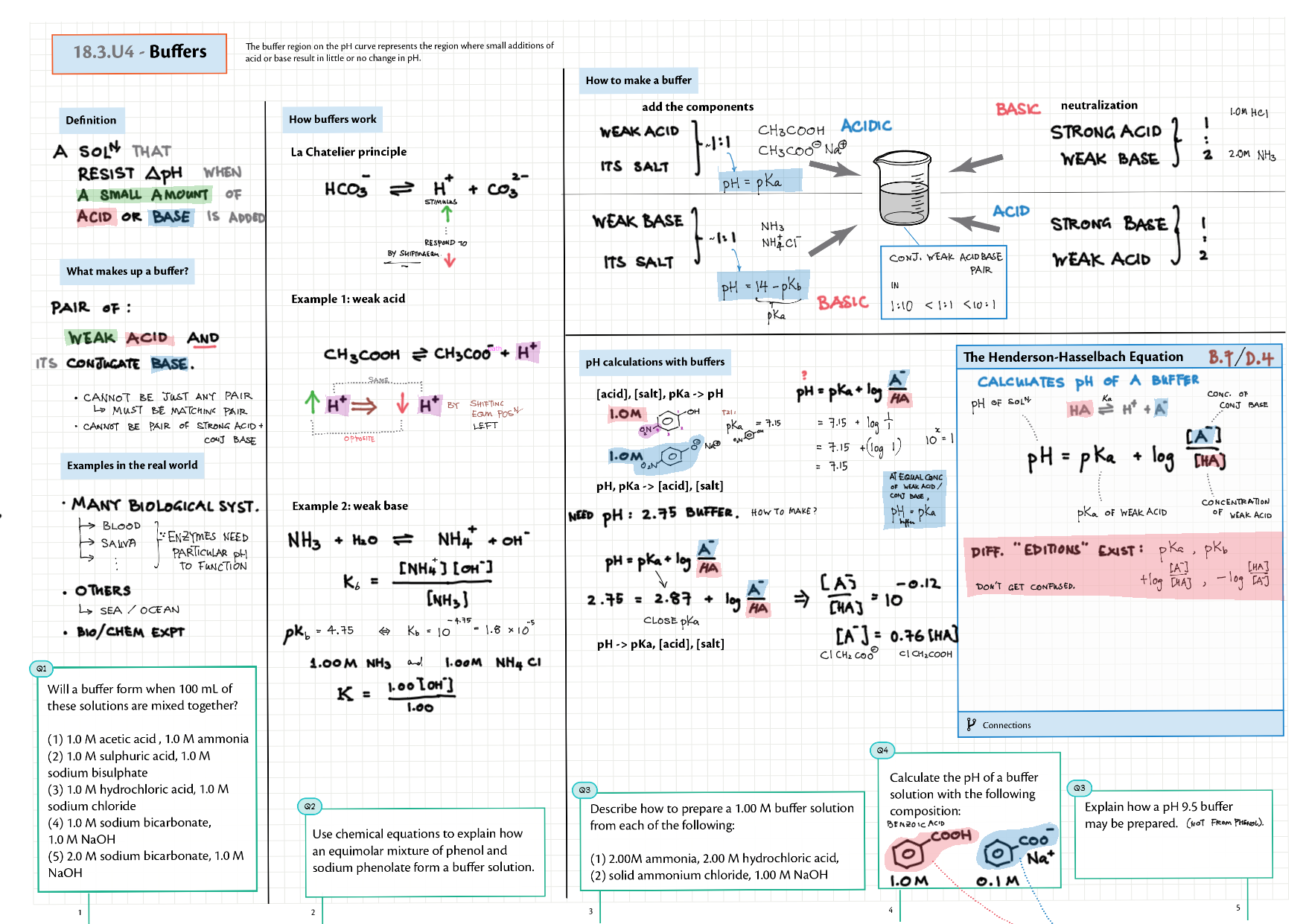
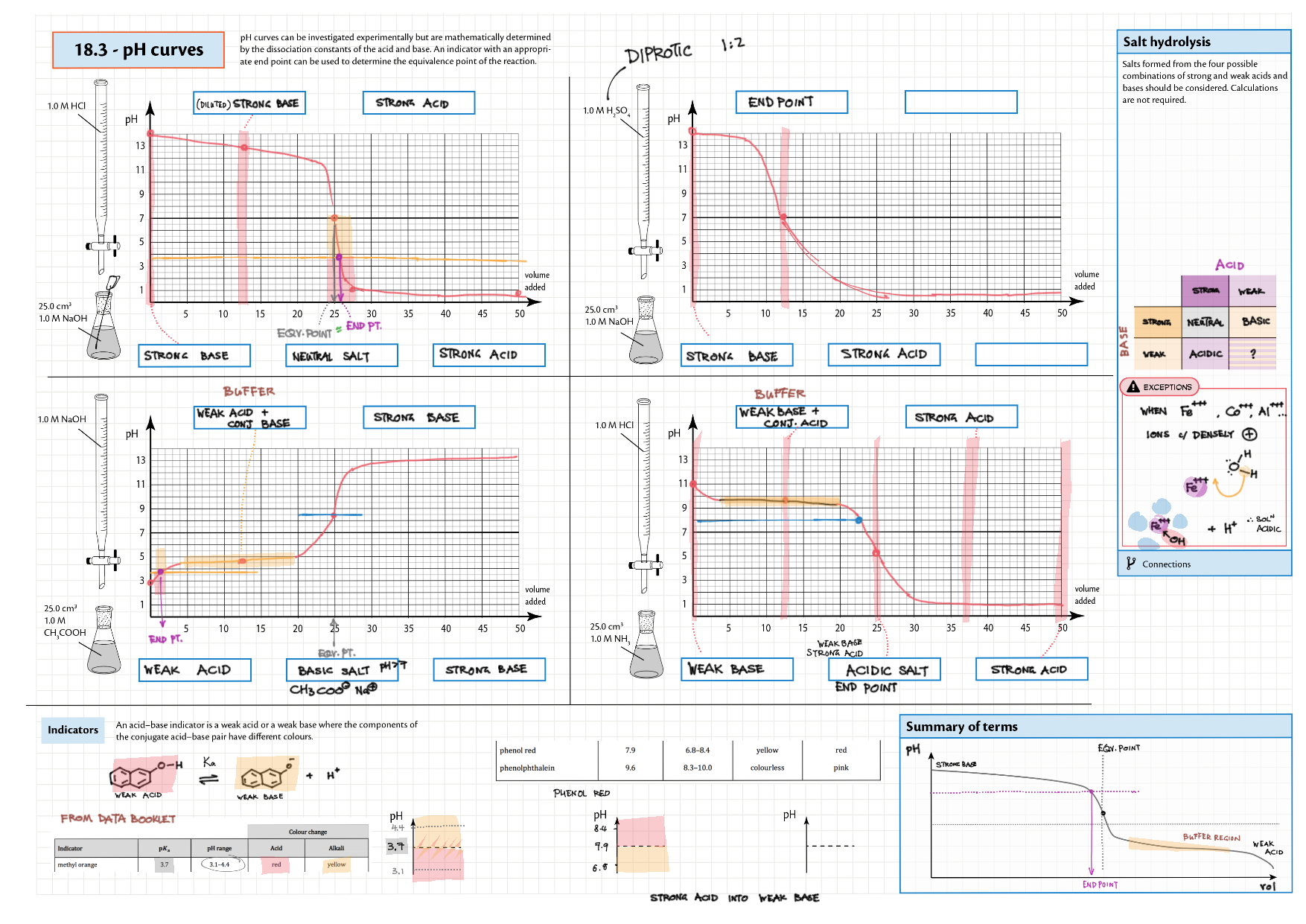
8/18 - Acids and Bases topics note 8 / 18 / 10 Definitions, Lewis acid, e- pushing 8 Properties of acids / bases, pH scale 8 Environmental concerns 18 Acid base calculations 18 pH curve calculations 18 Buffers 18 Salt hydrolysis, indicators, pH curve variations
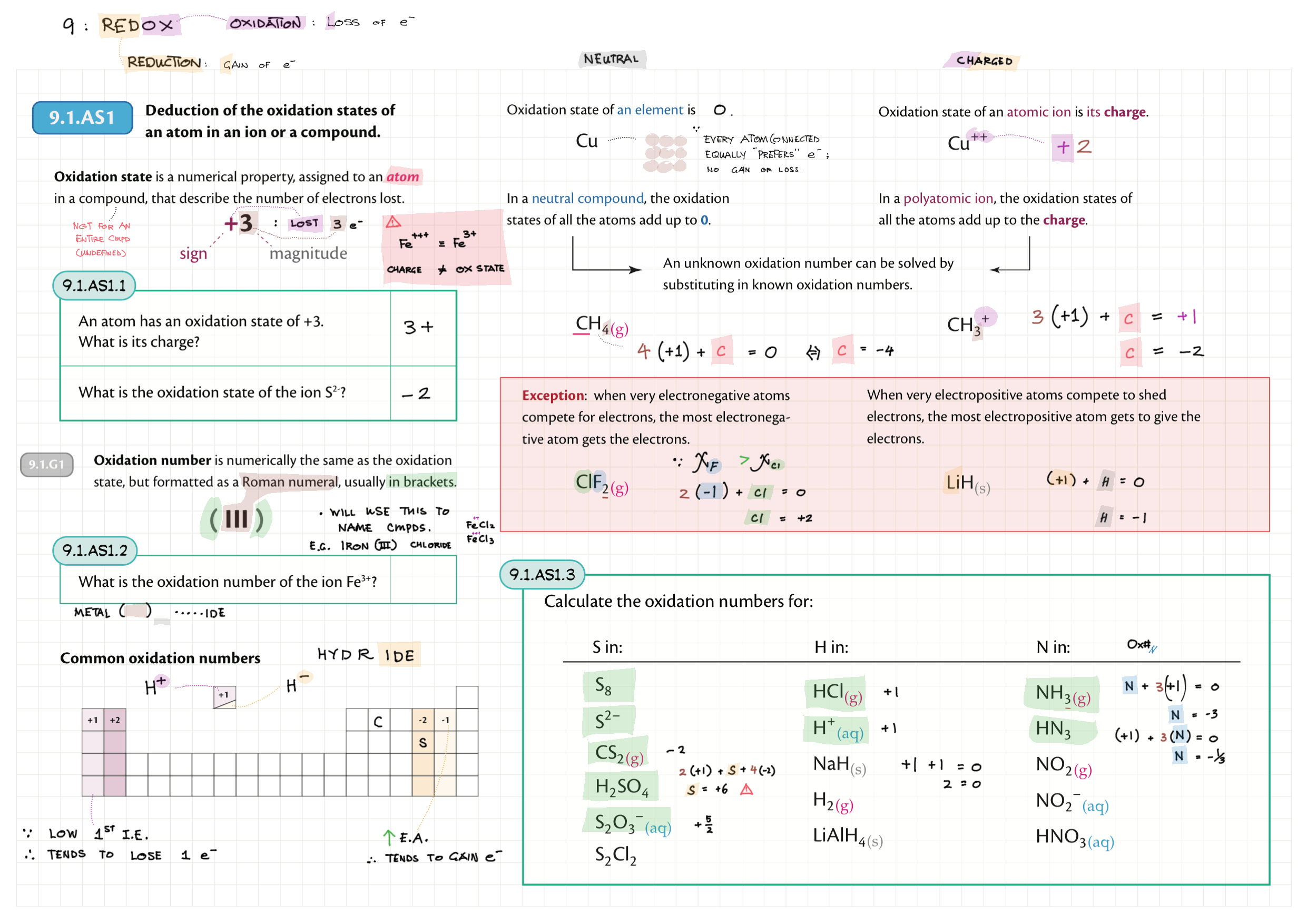
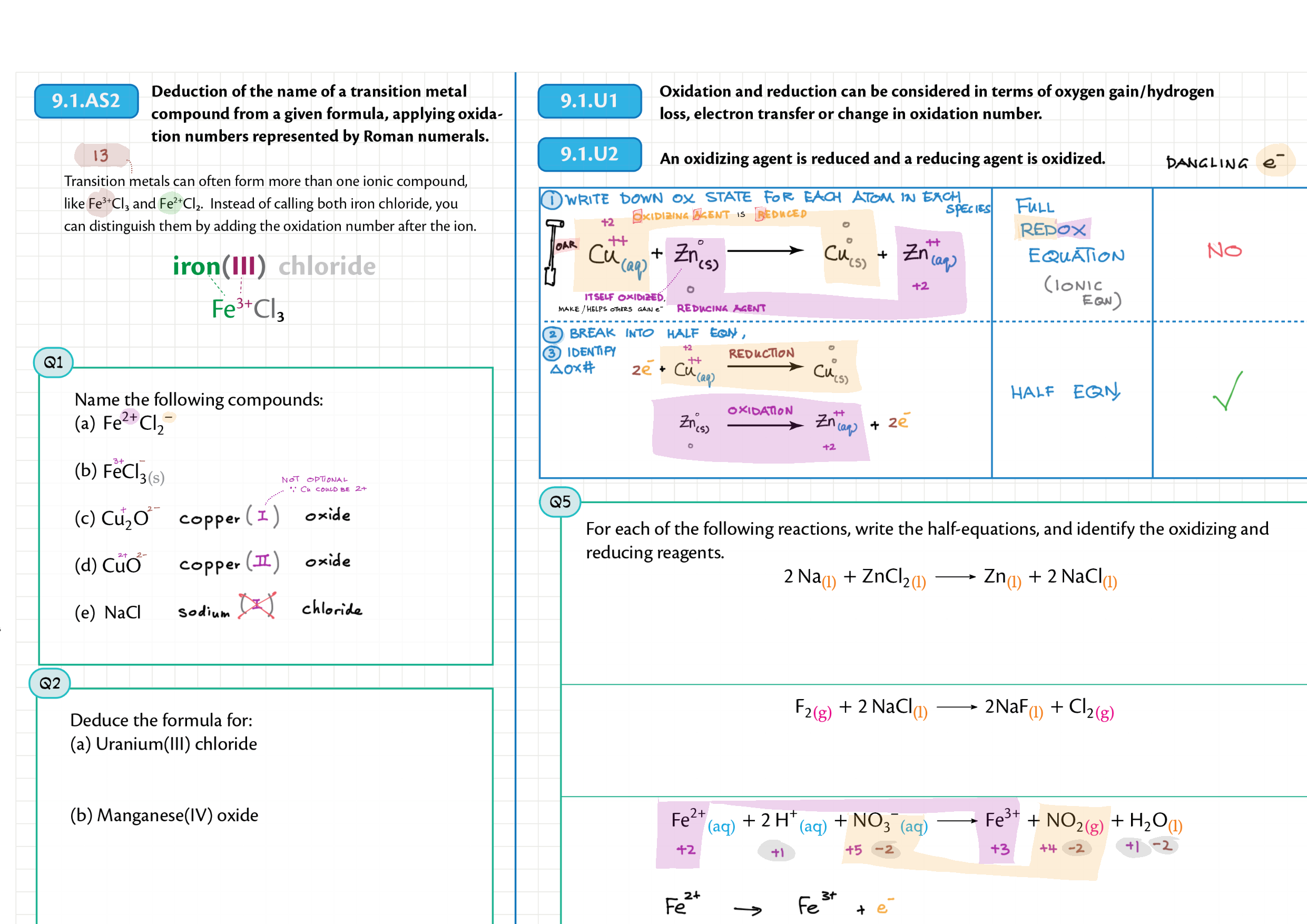
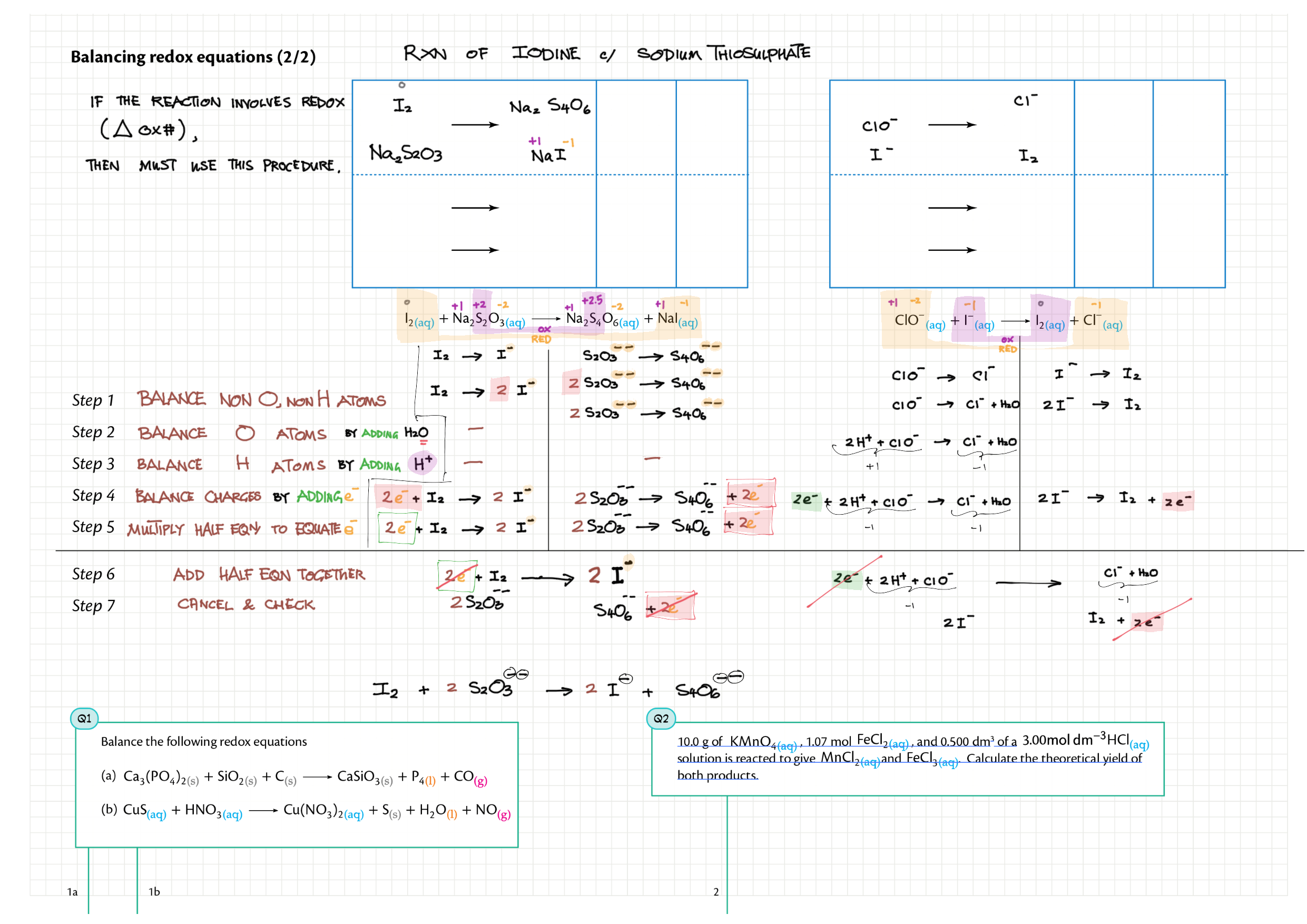
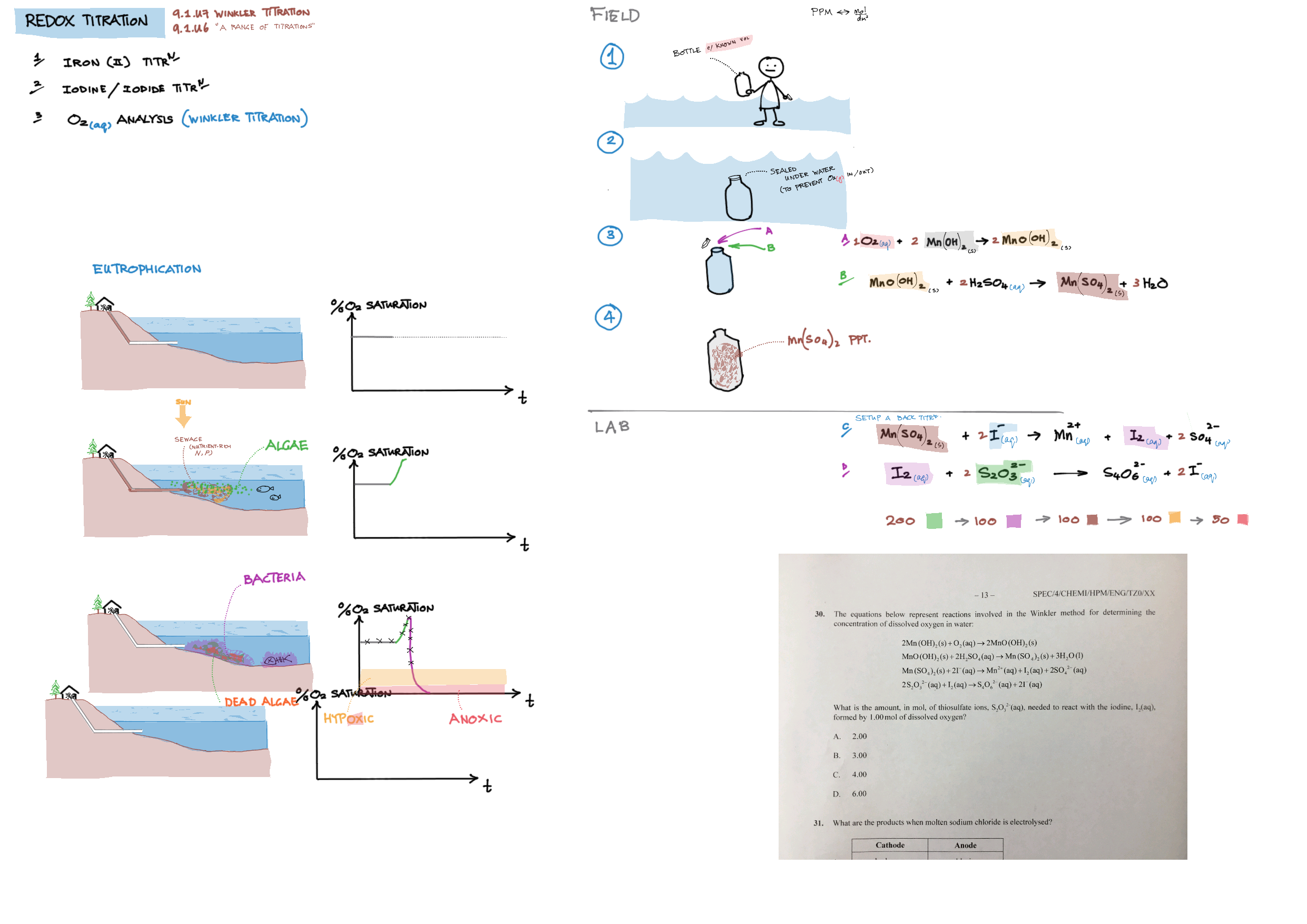
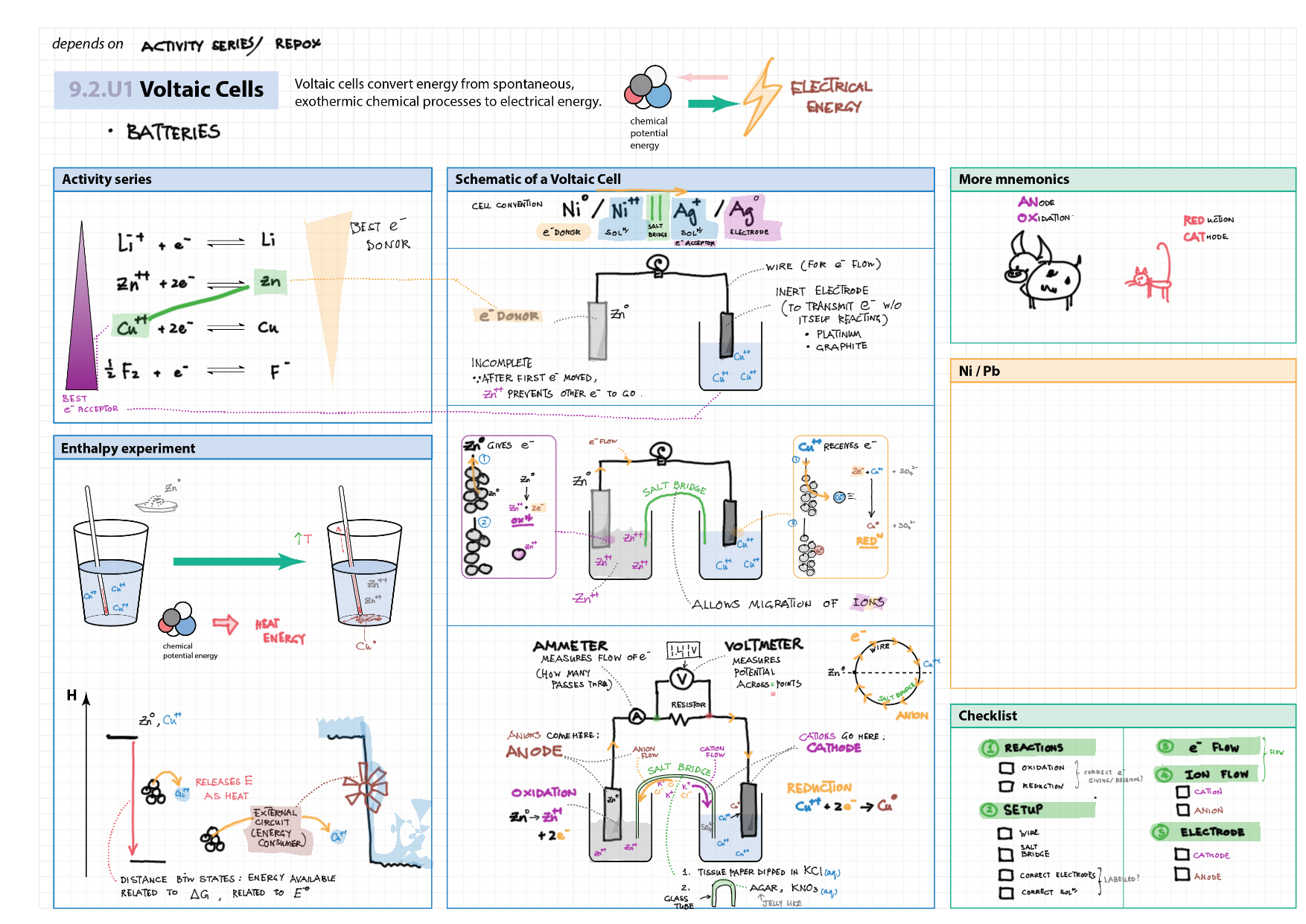
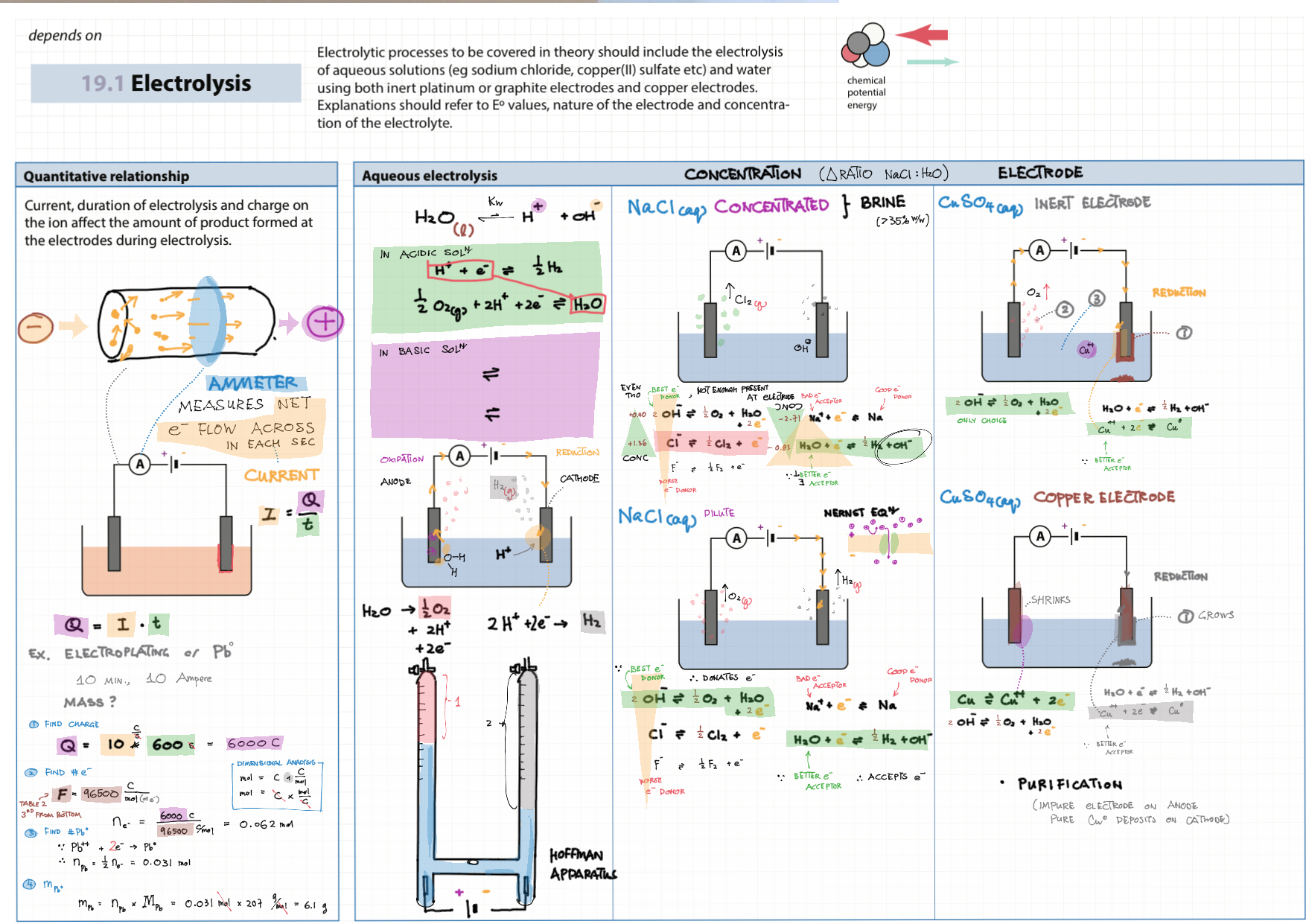
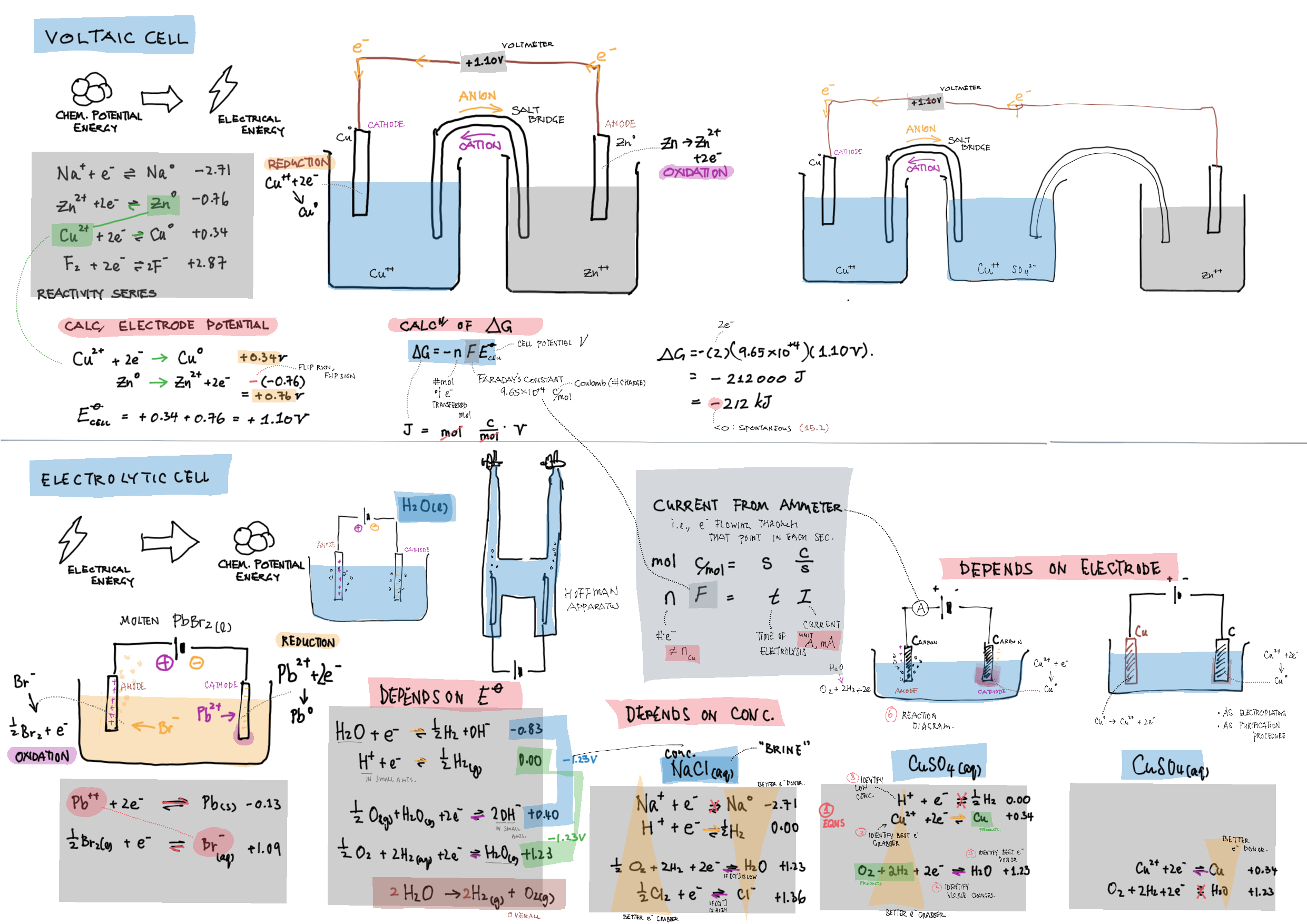
9/19 - Redox topics note 9 Oxidation number/states 9 Redox agents, half equations, nomenclature 9 Balancing redox equations 9 Redox titrations 9 Voltaic cells 9 / 19 Electrolytic cell / electrolysis in aq solutions 19 Cell potential & current calc
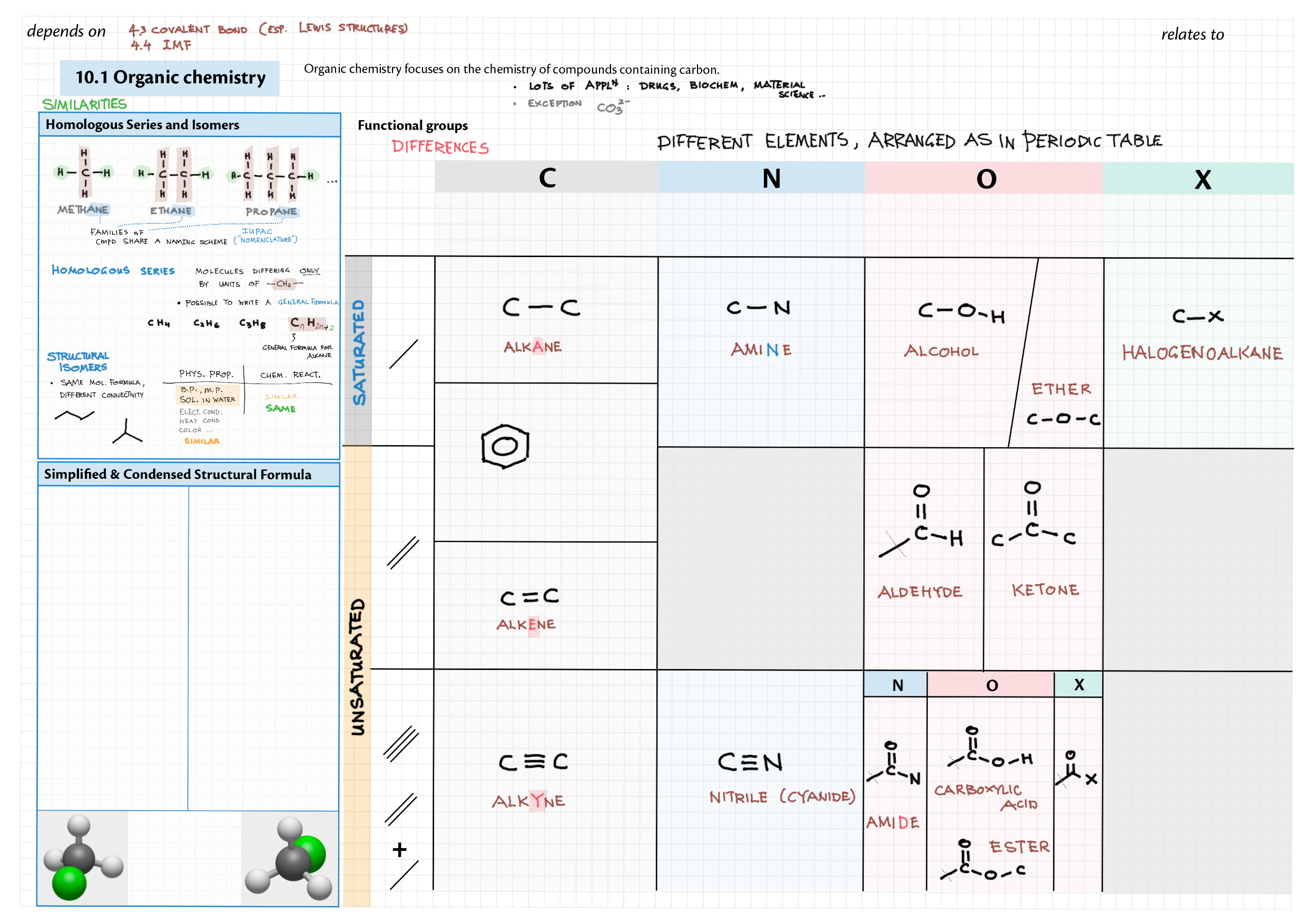
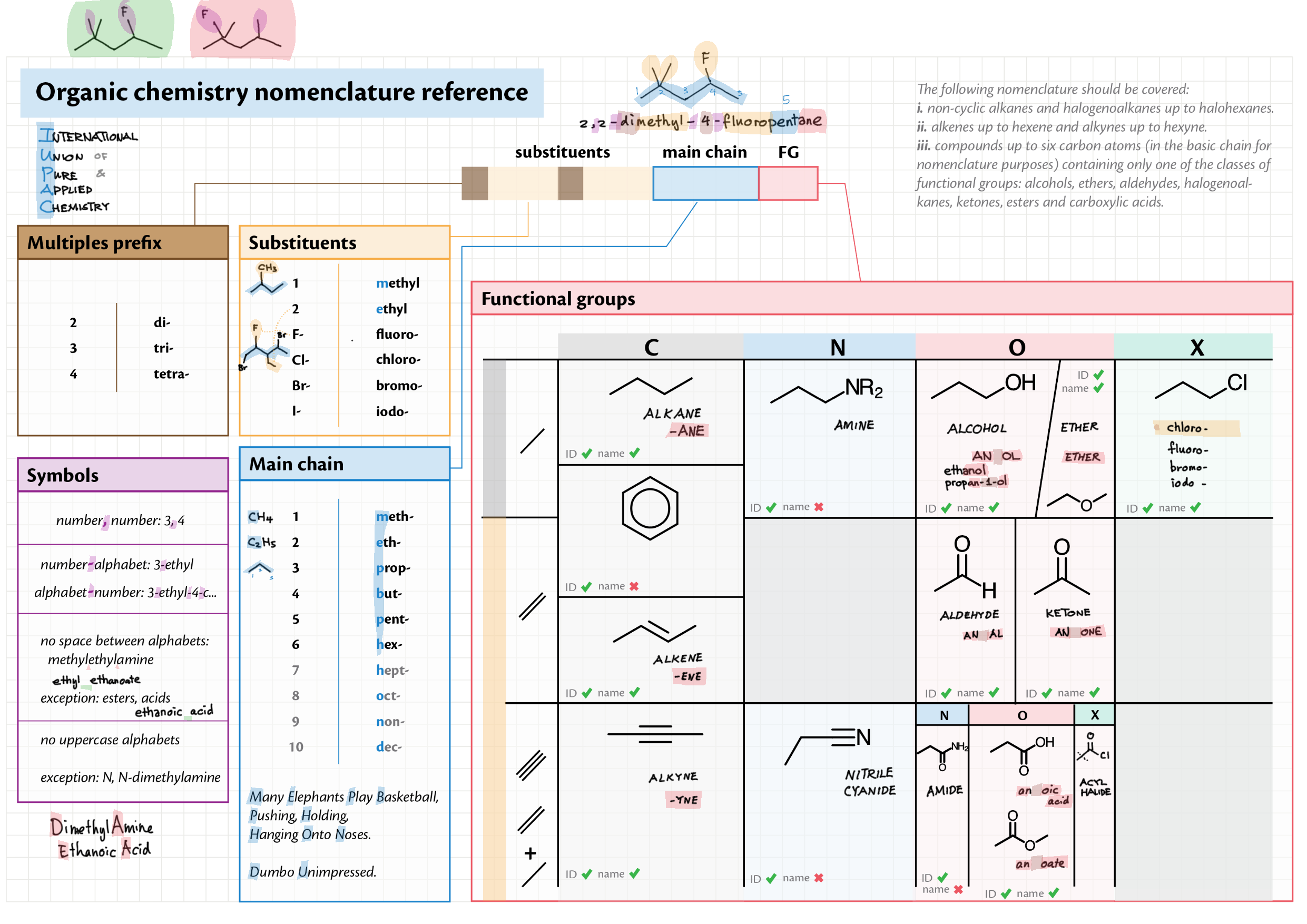
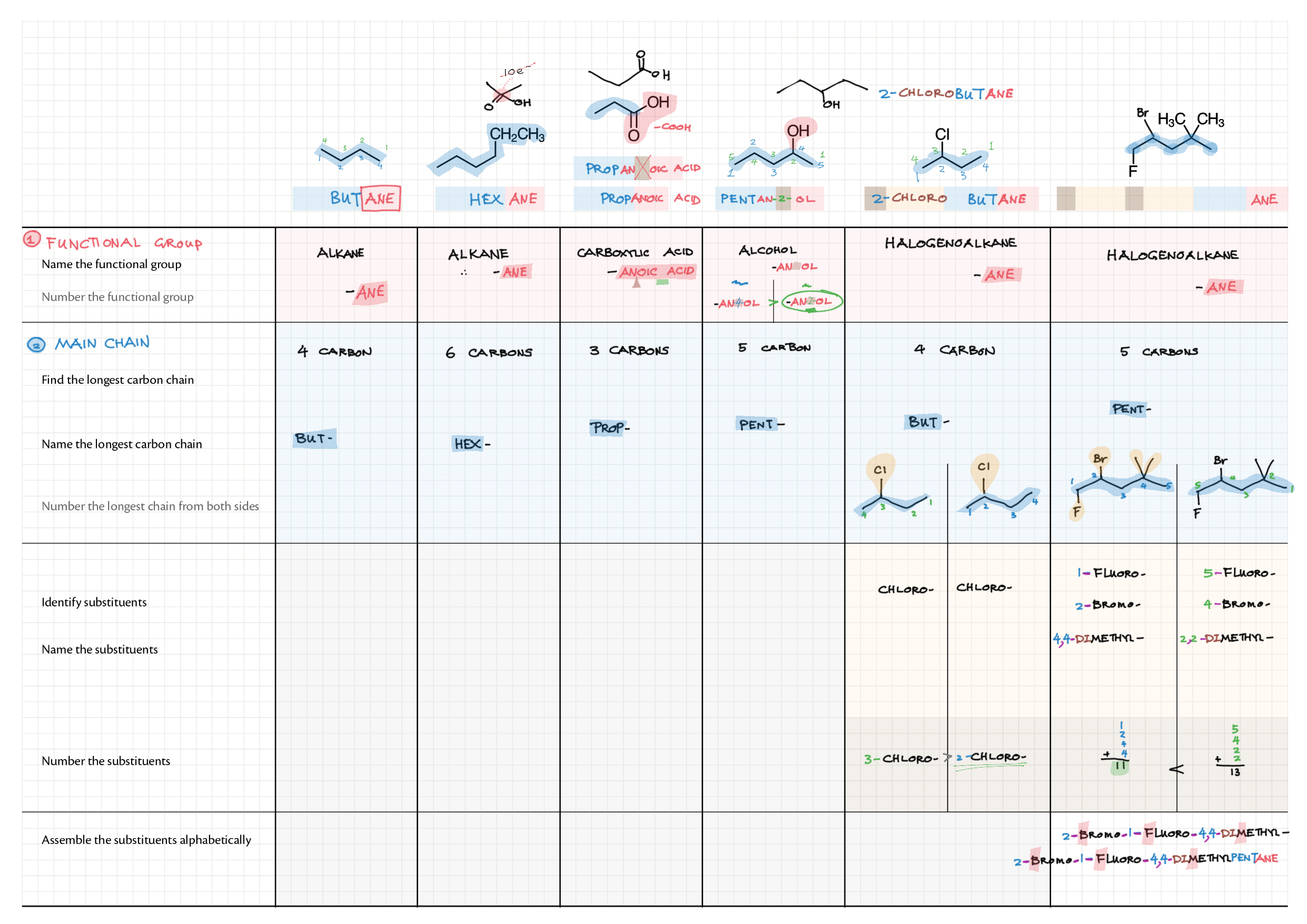
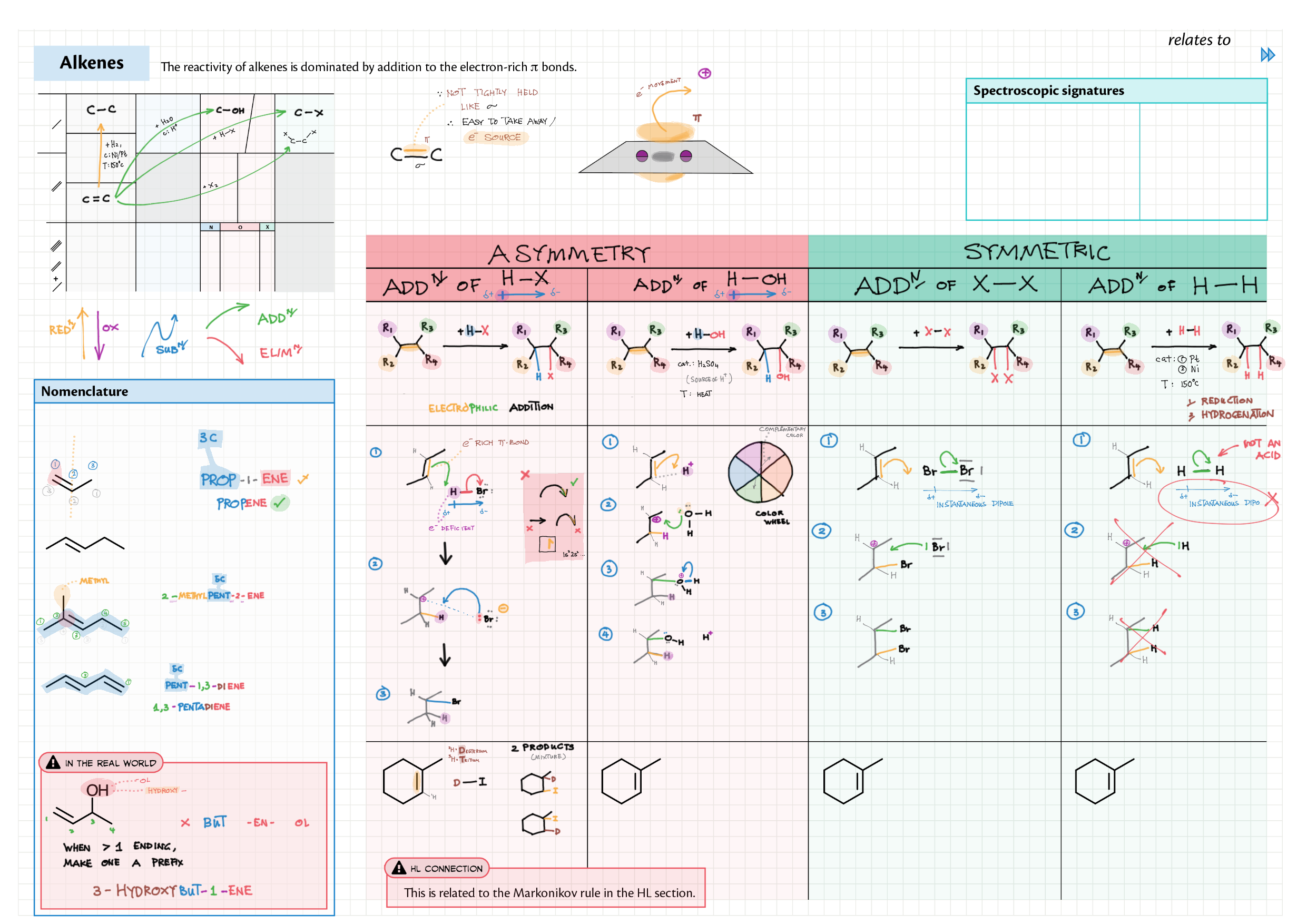
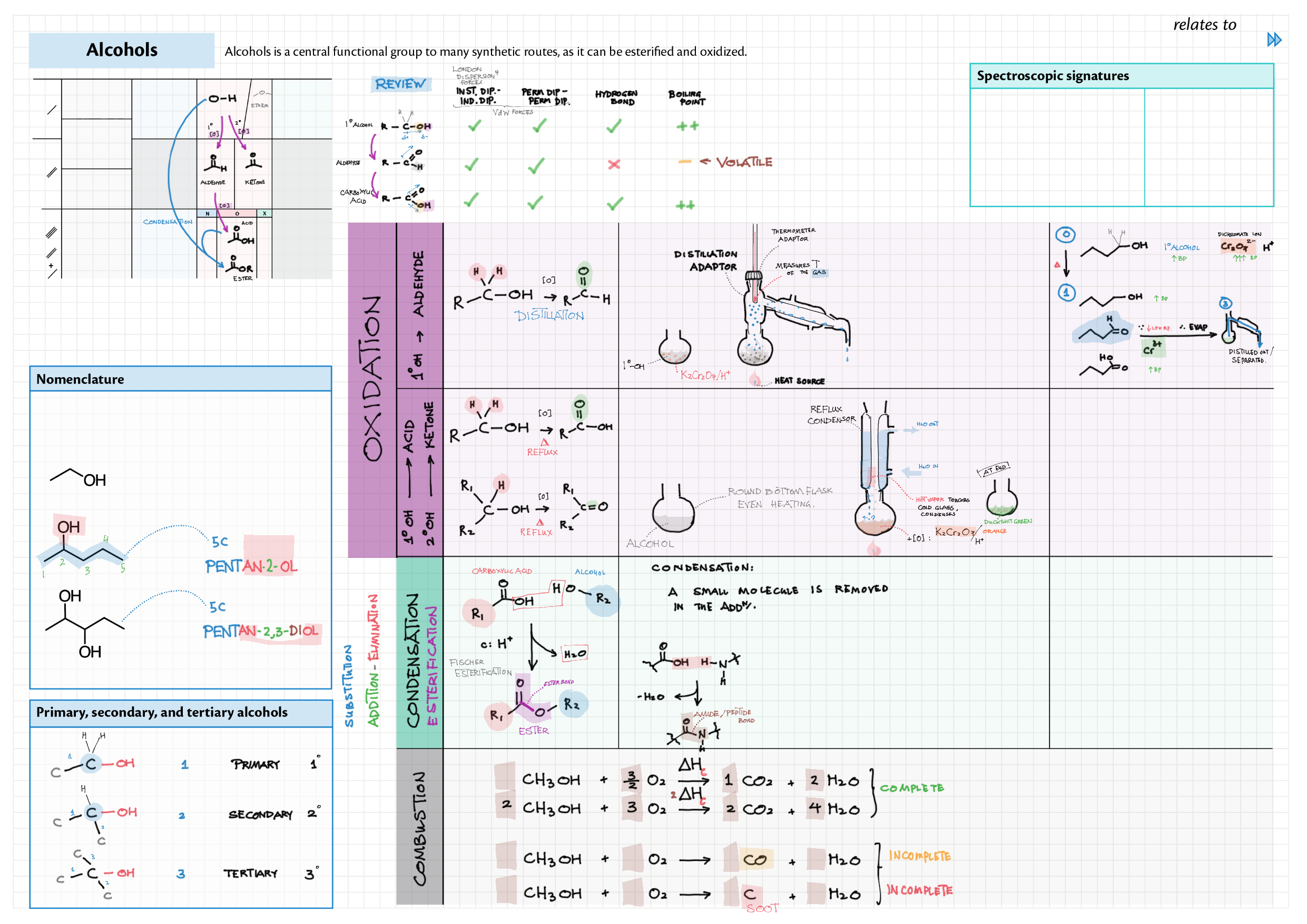
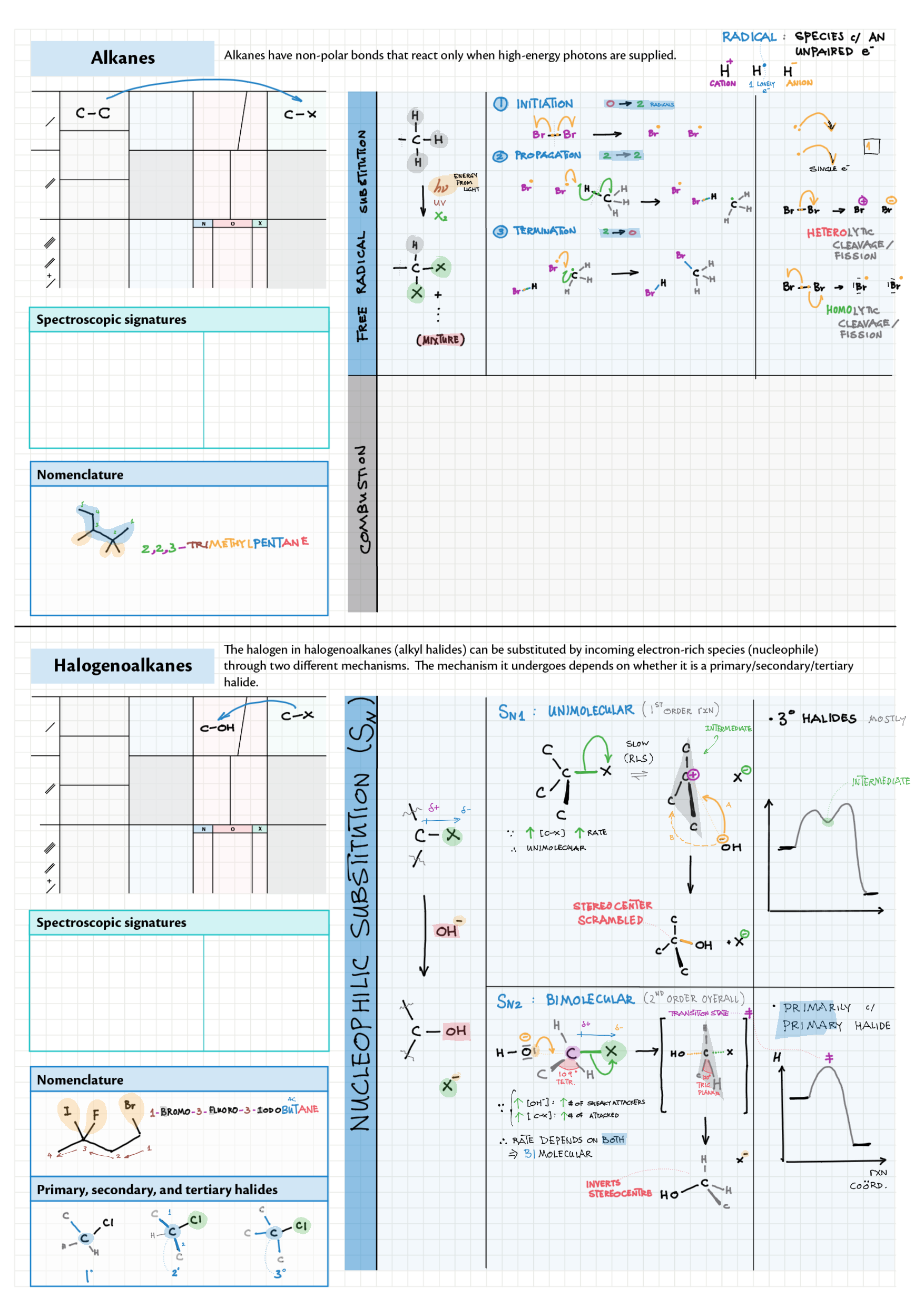
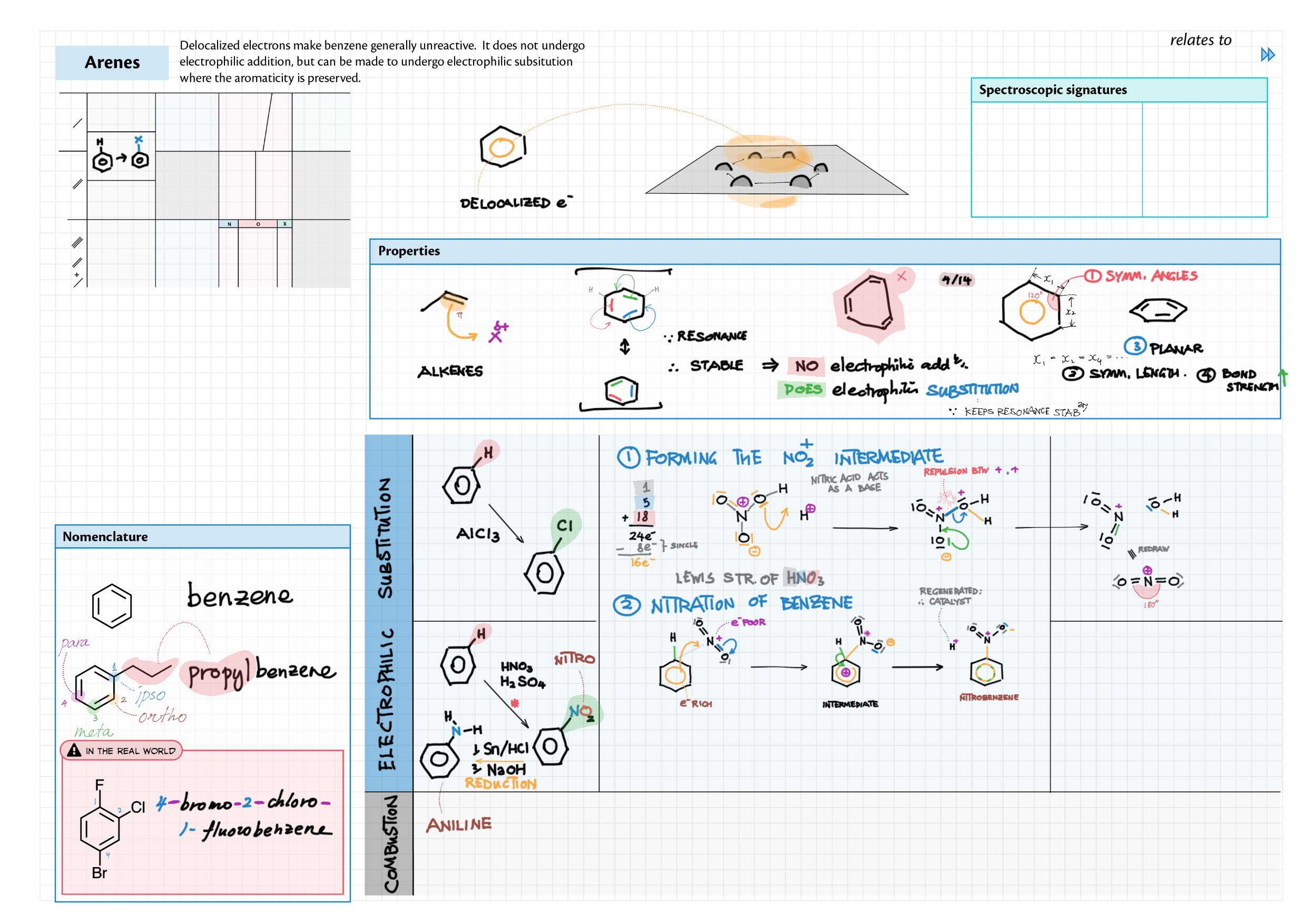
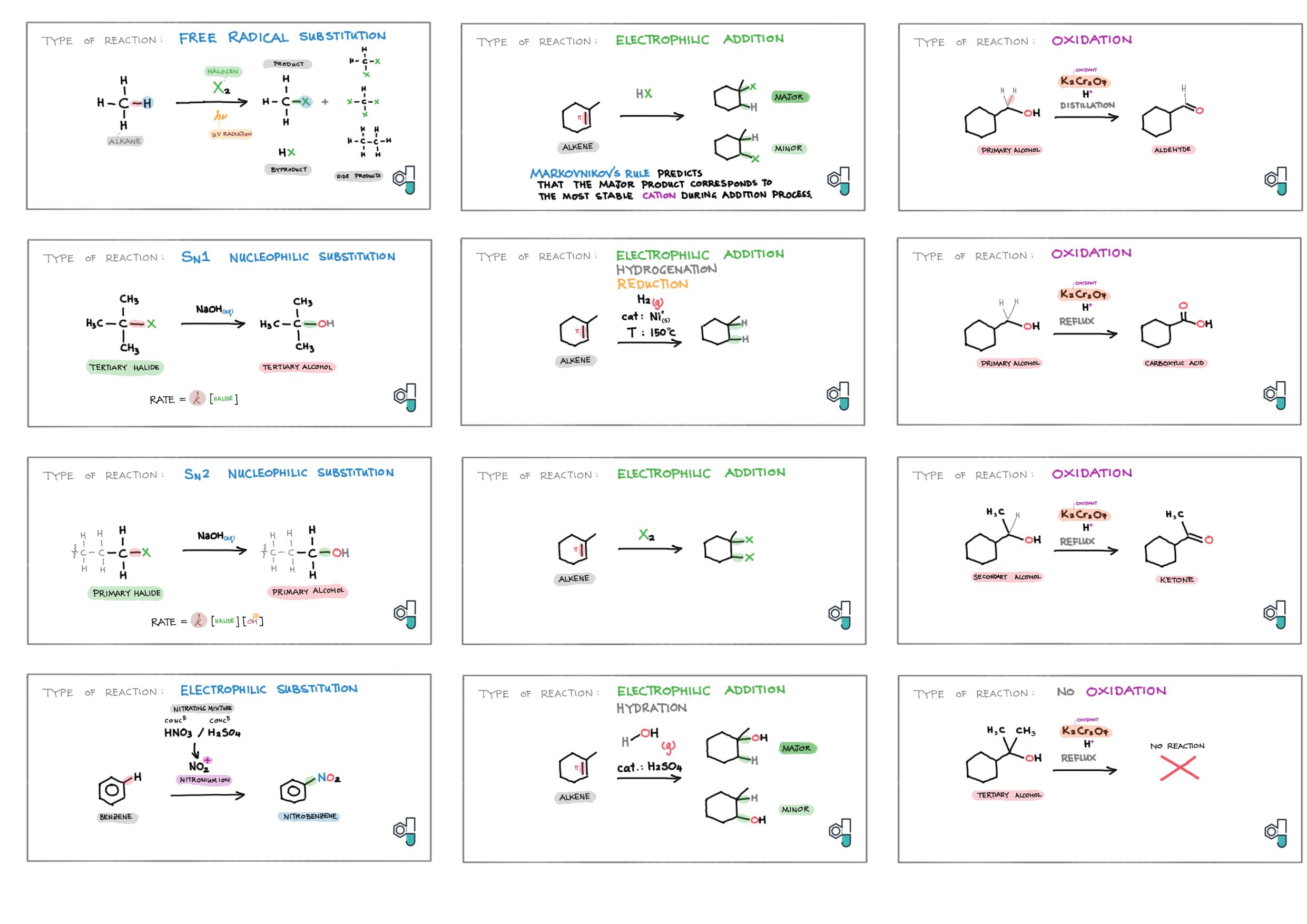
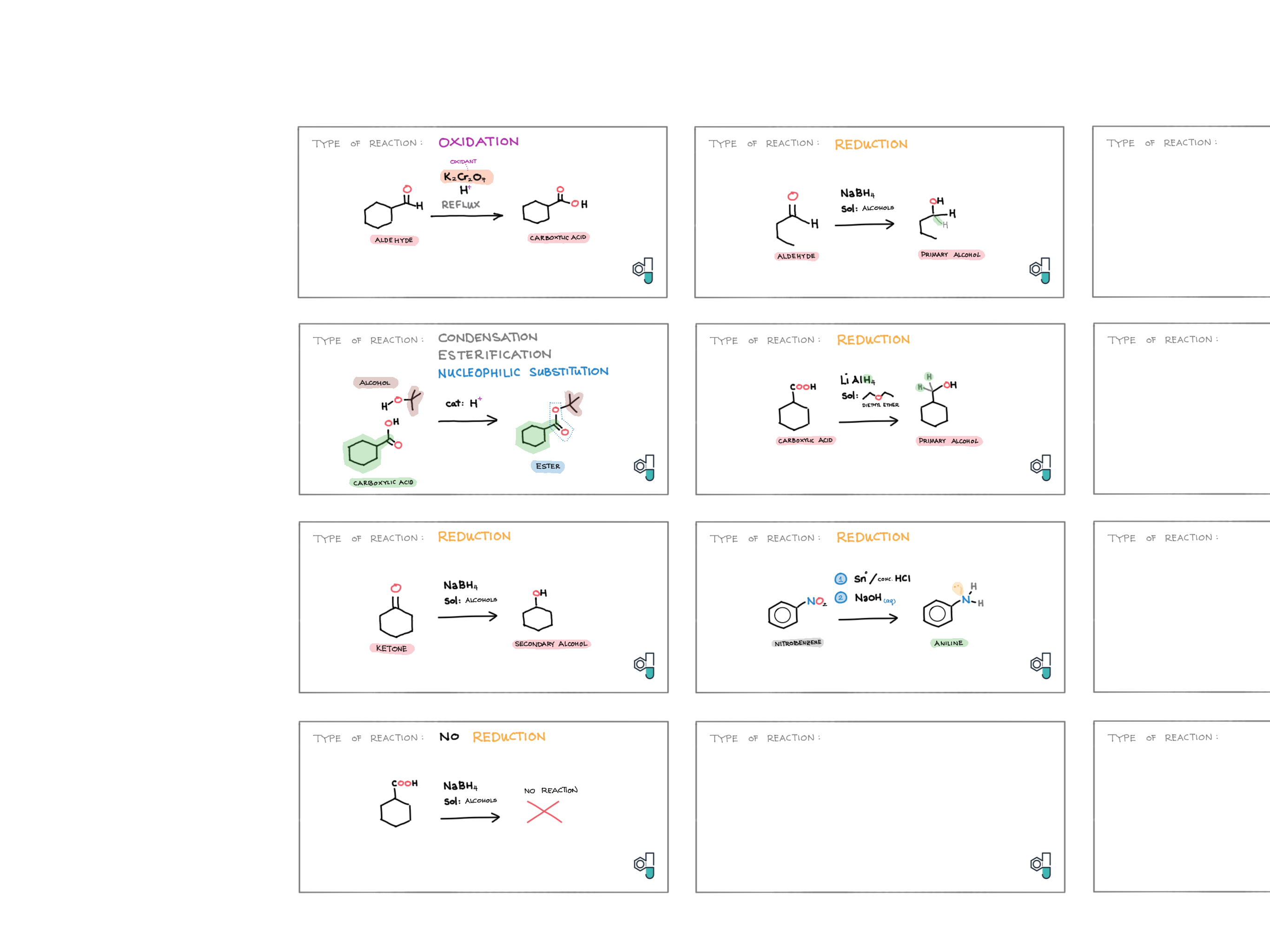
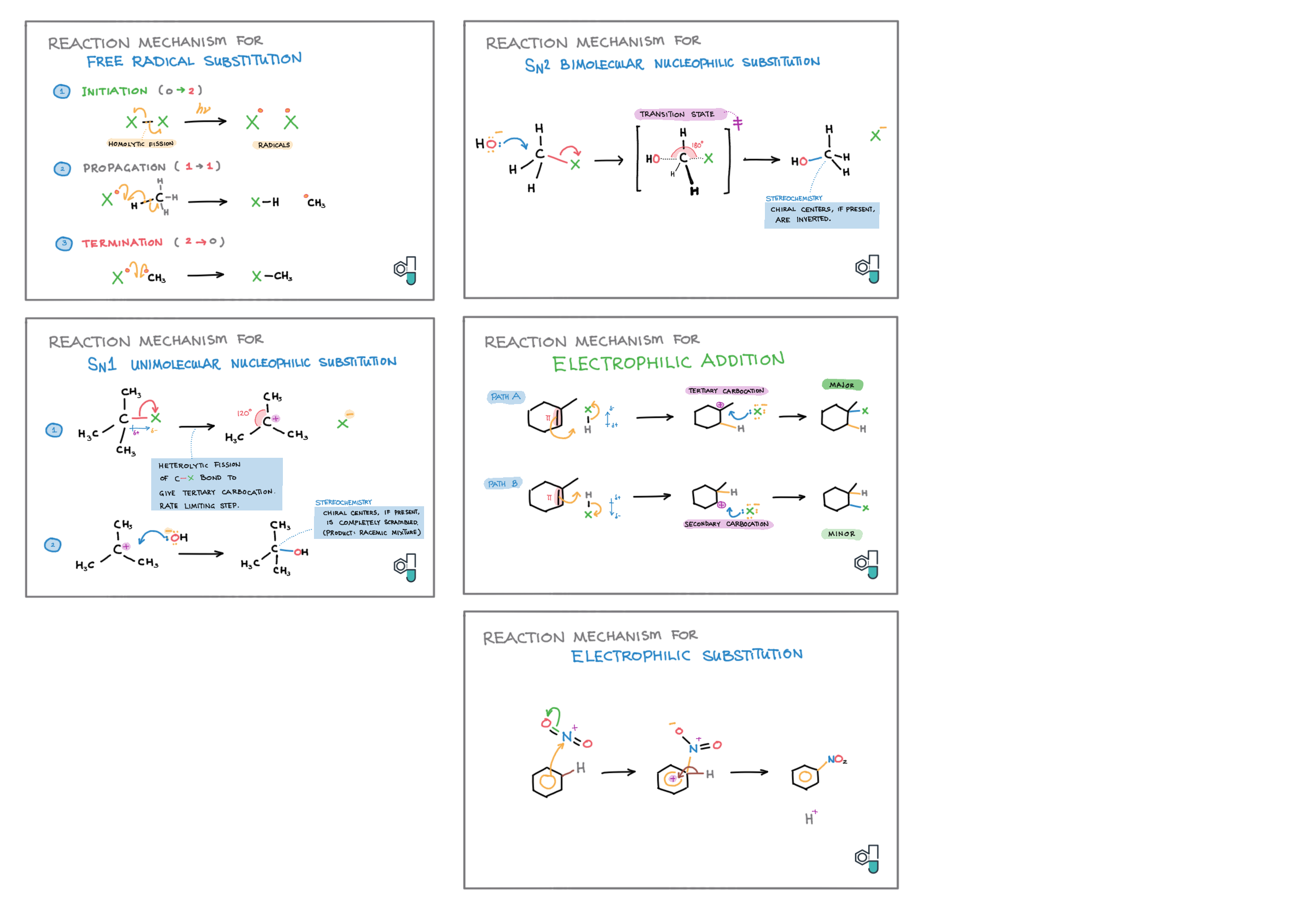
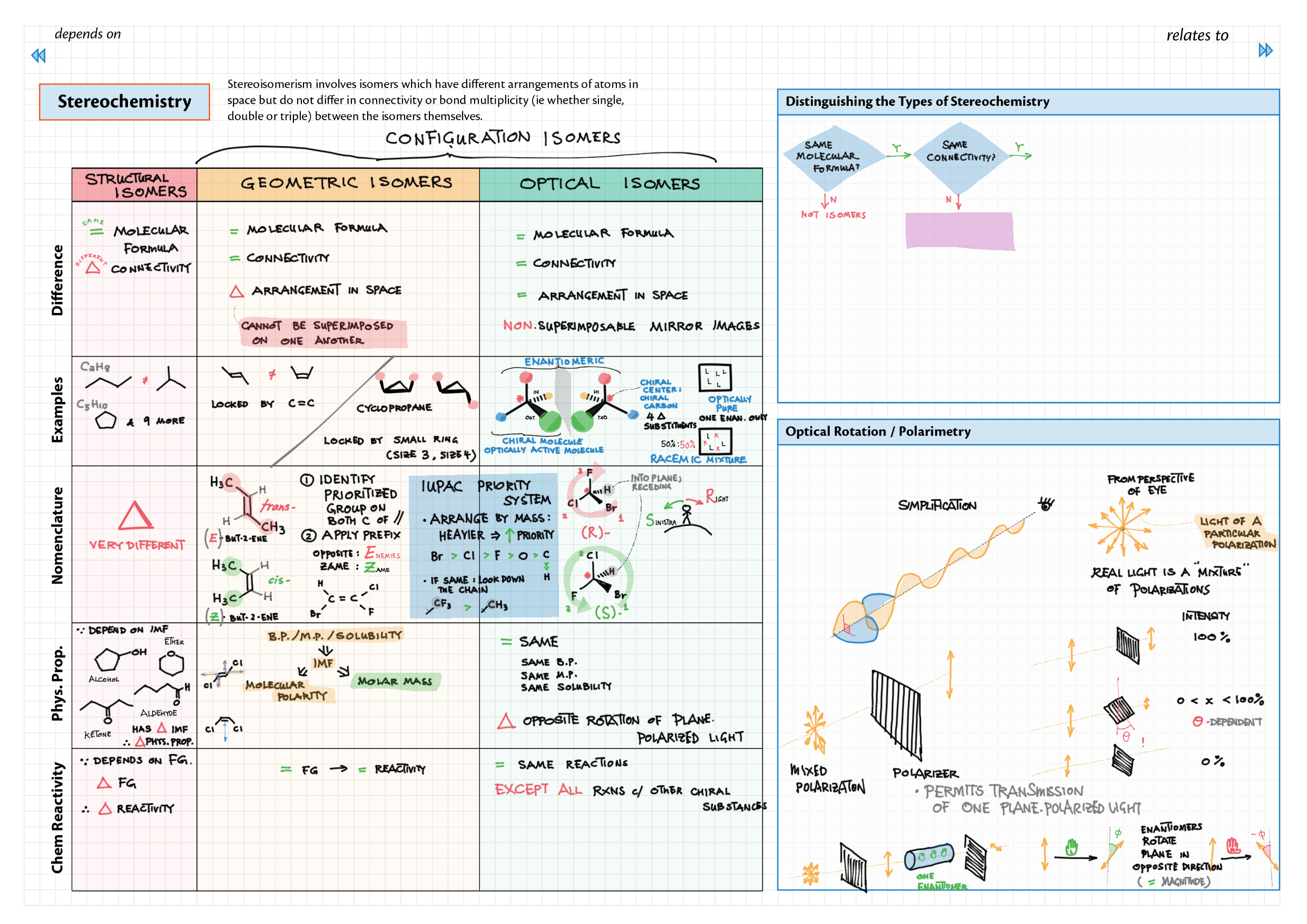
10/20 - Organic chemistry topics note 10 Isomers / homologous series, functional group names 10 IUPAC nomenclature reference 10 Step-by-step IUPAC nomenclature 1 10 Alkene 10 Alcohol 10 Alkane, halogenoalkane 20 Arenes 20 Reaction summary 1 10 / 20 Reaction summary 2 20 Mechanism summary 20 Stereochemistry
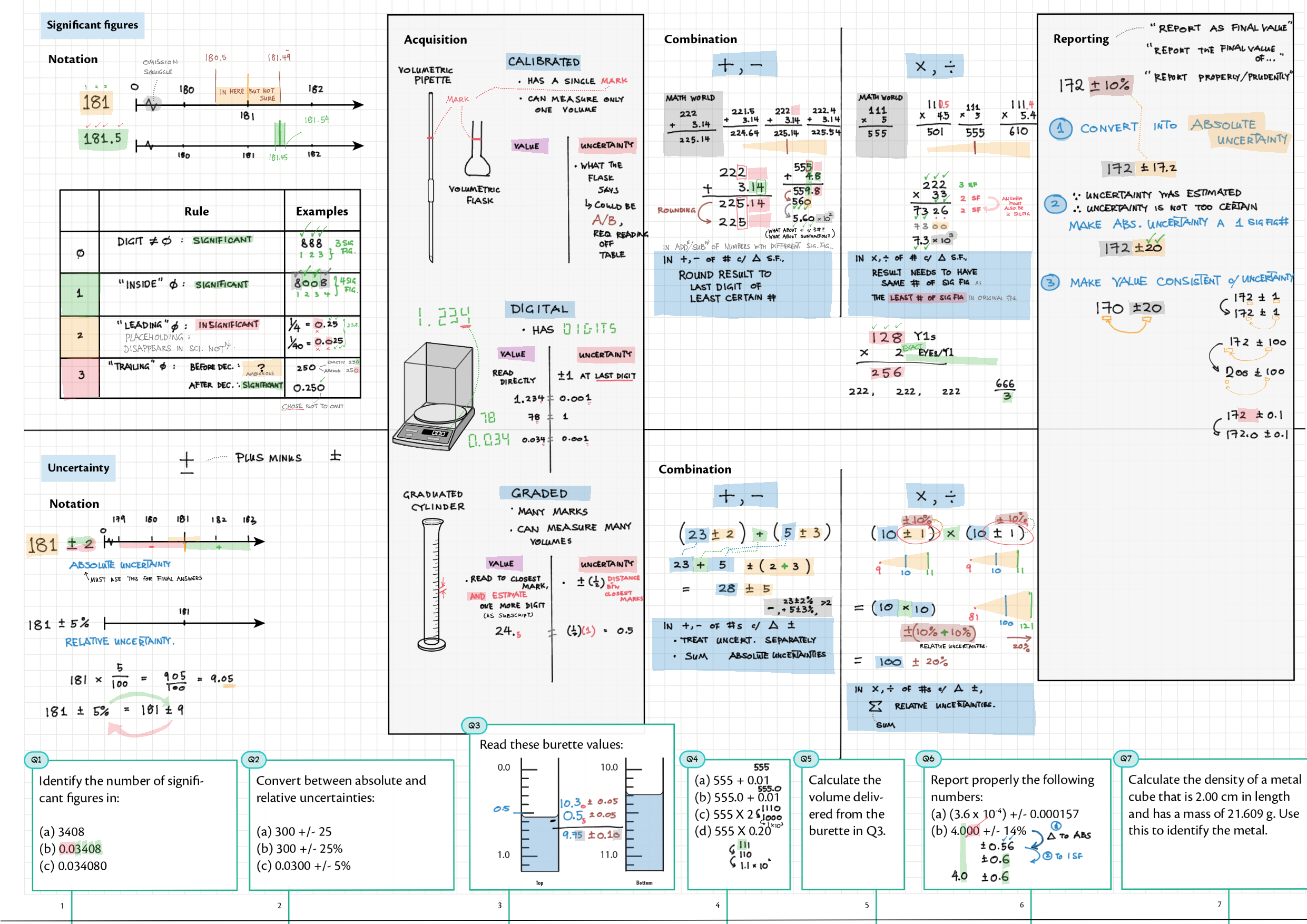
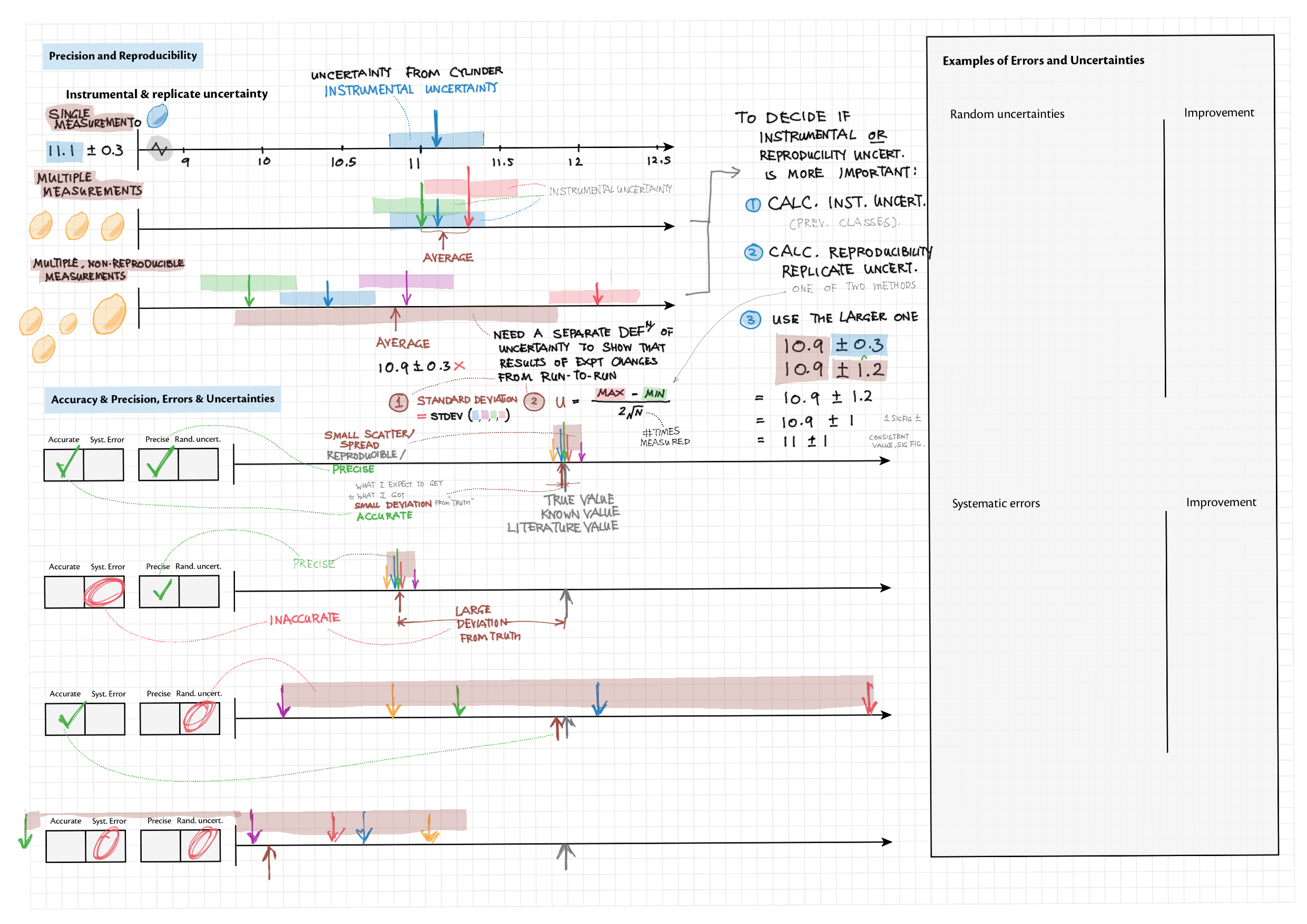
11A - Measurements topics note 11A Significant figures and uncertainty 11A Uncertainty/reproducibility, precision and accuracy, systematic error
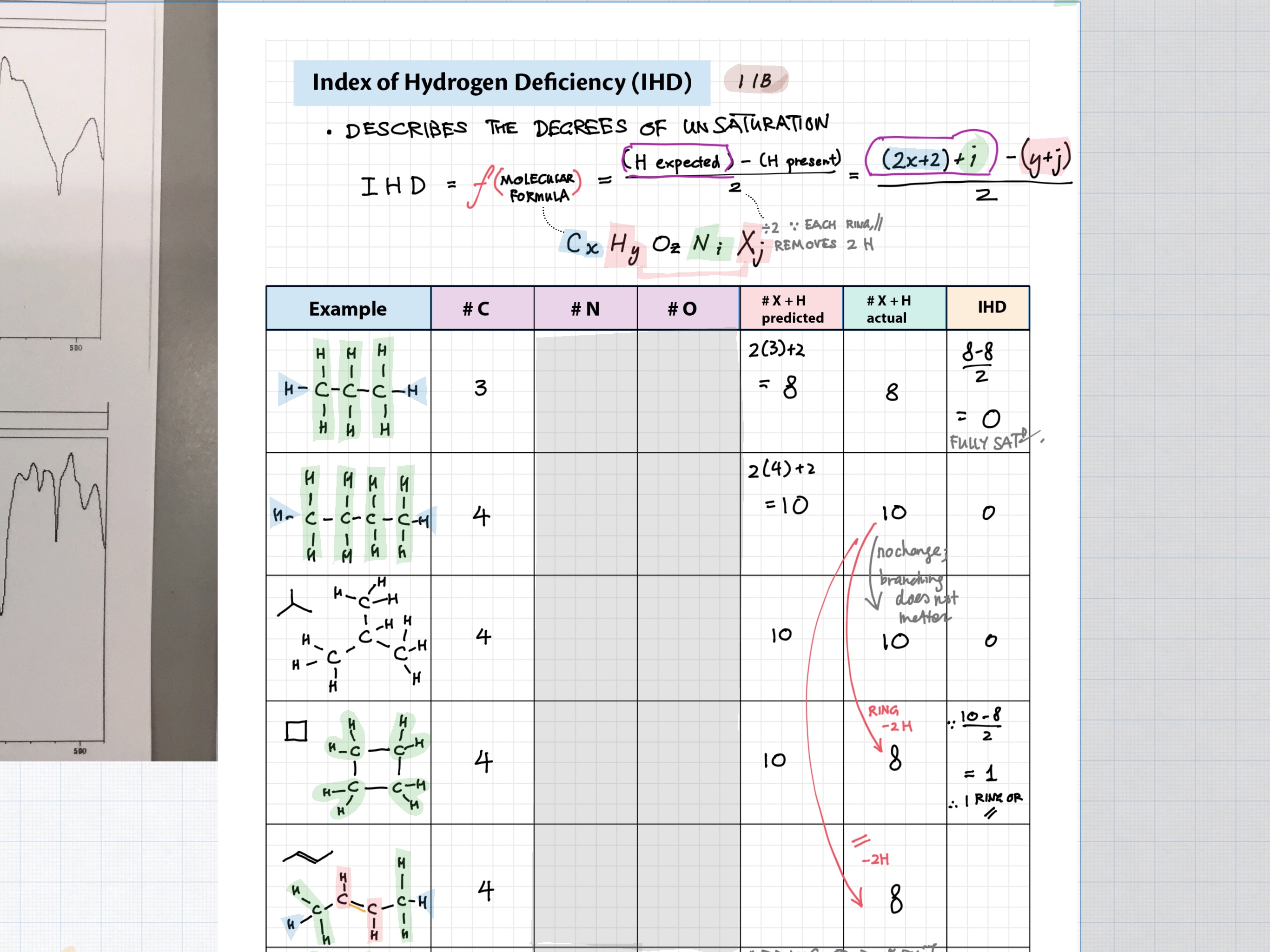
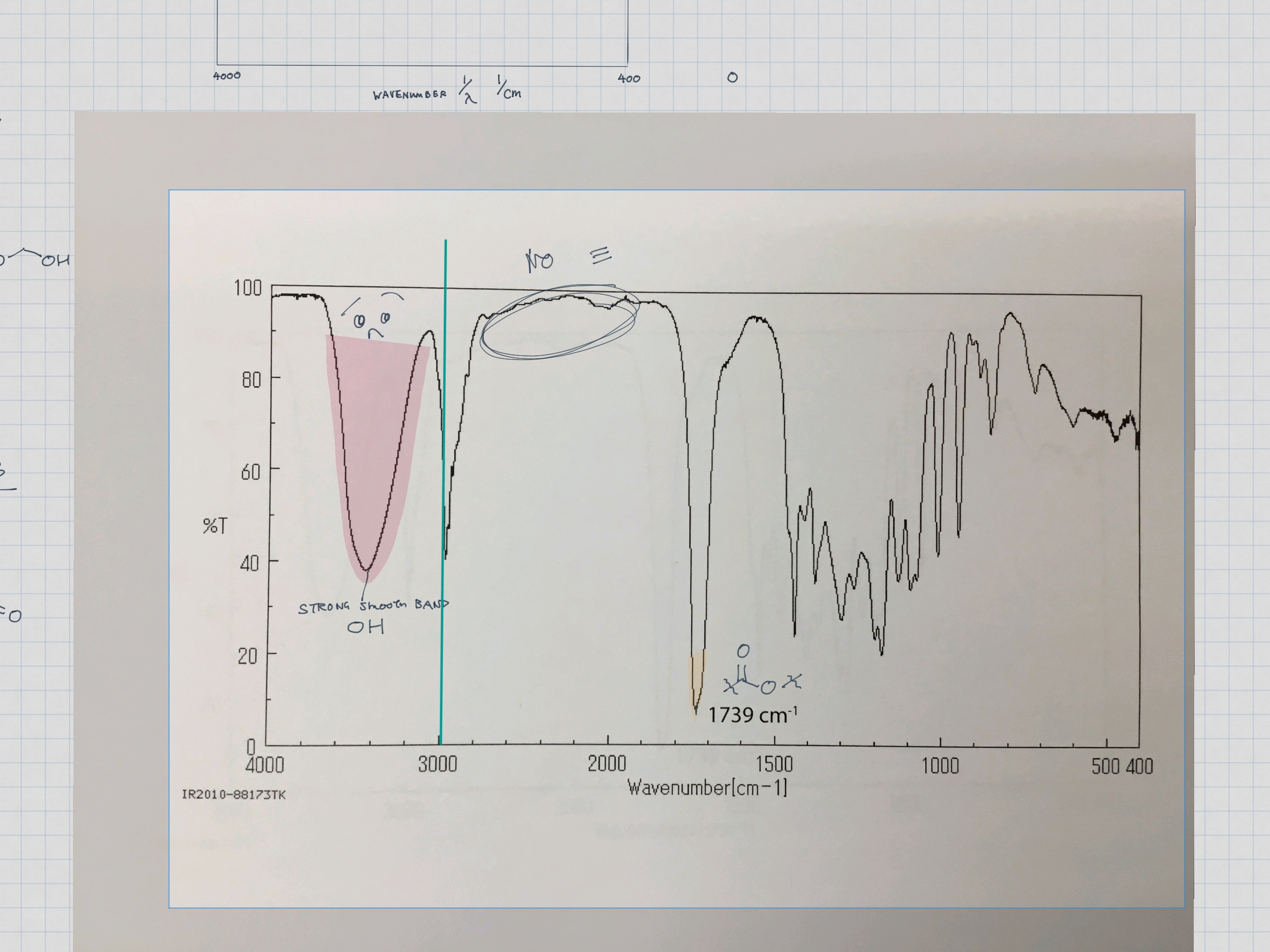
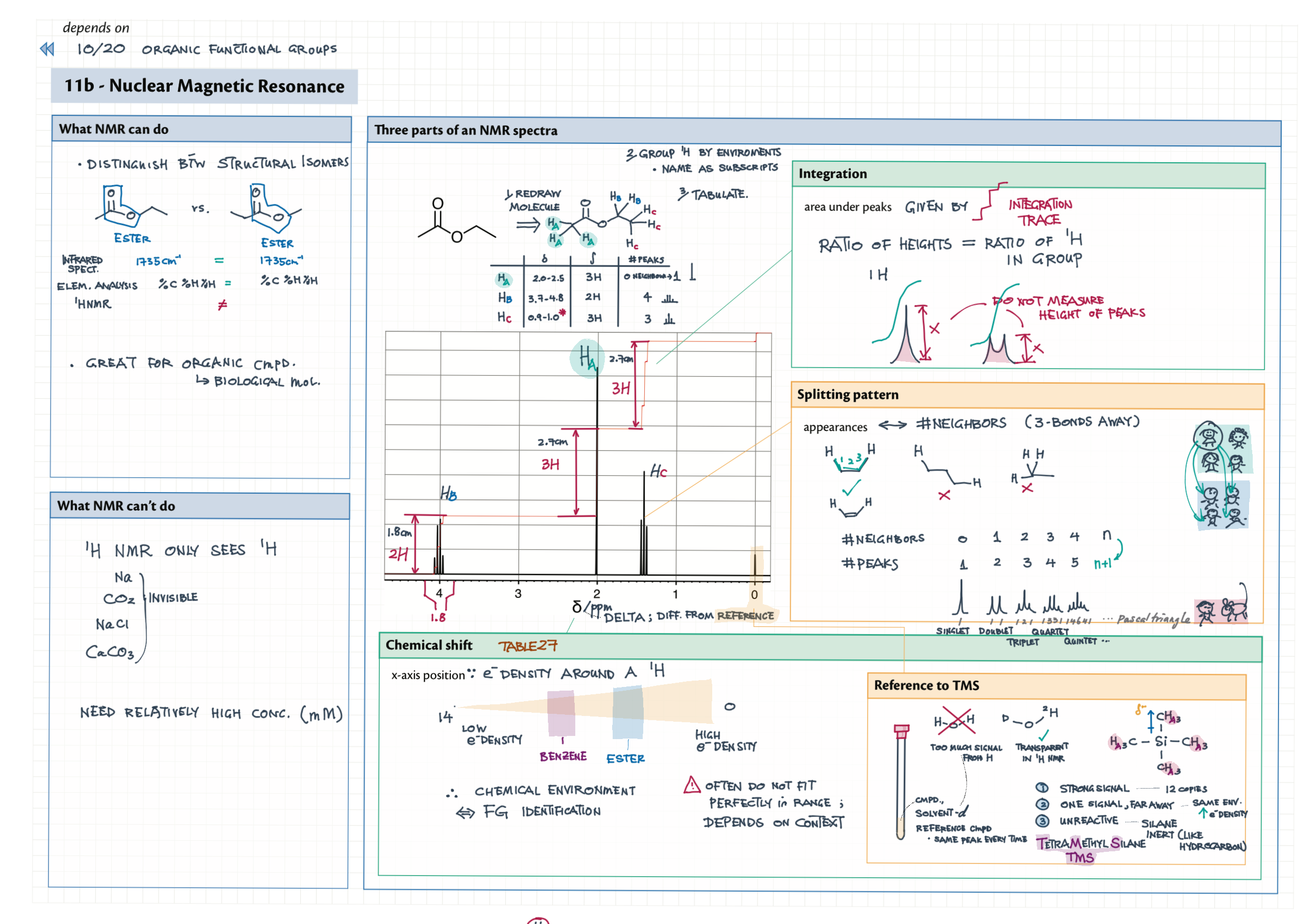
11B / 21 - Spectroscopy of Organic Compounds topics note 11B Index of hydrogen deficiency 11B IR spectra interpretation example 11B / 21 1 H NMR interpretation
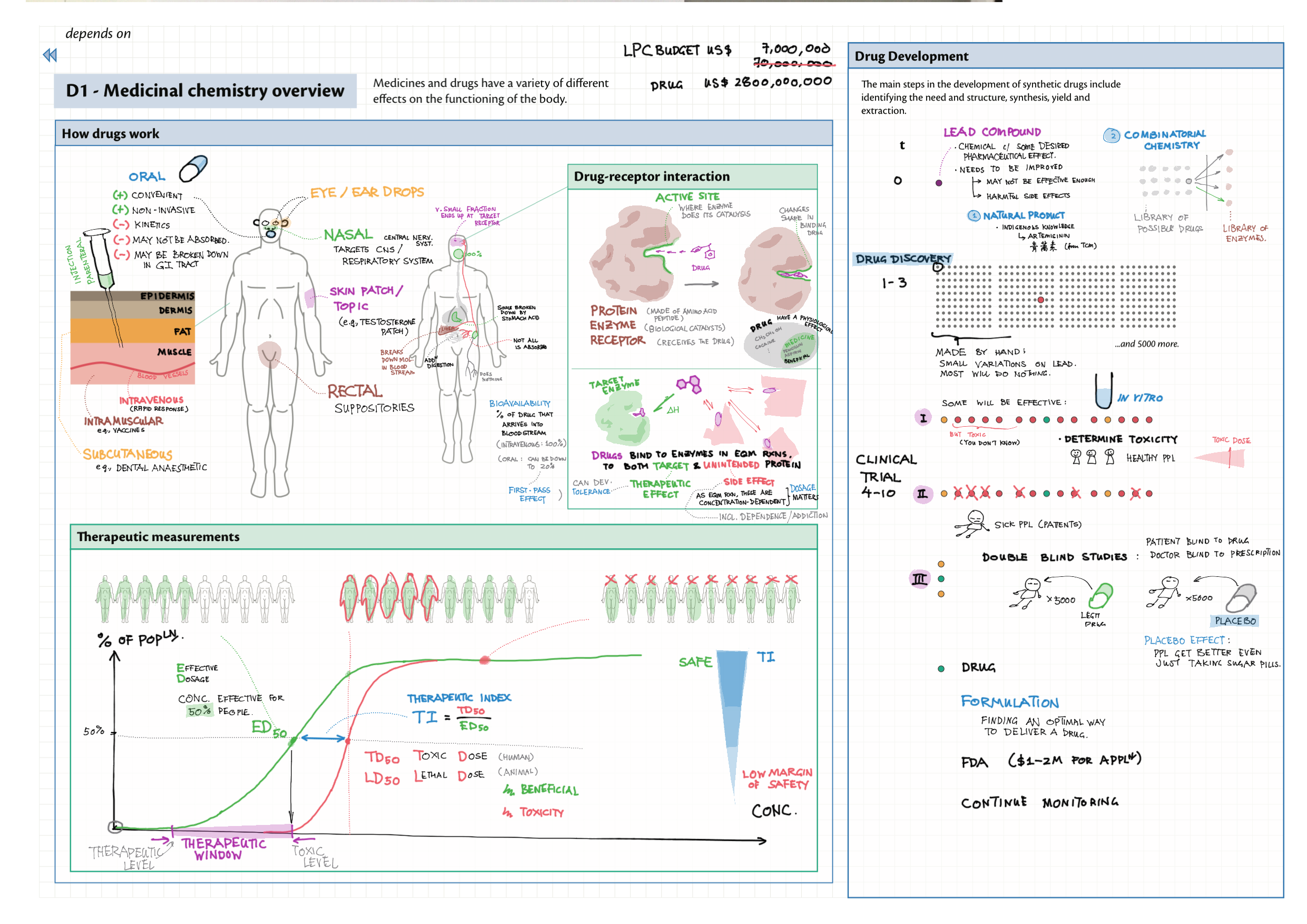
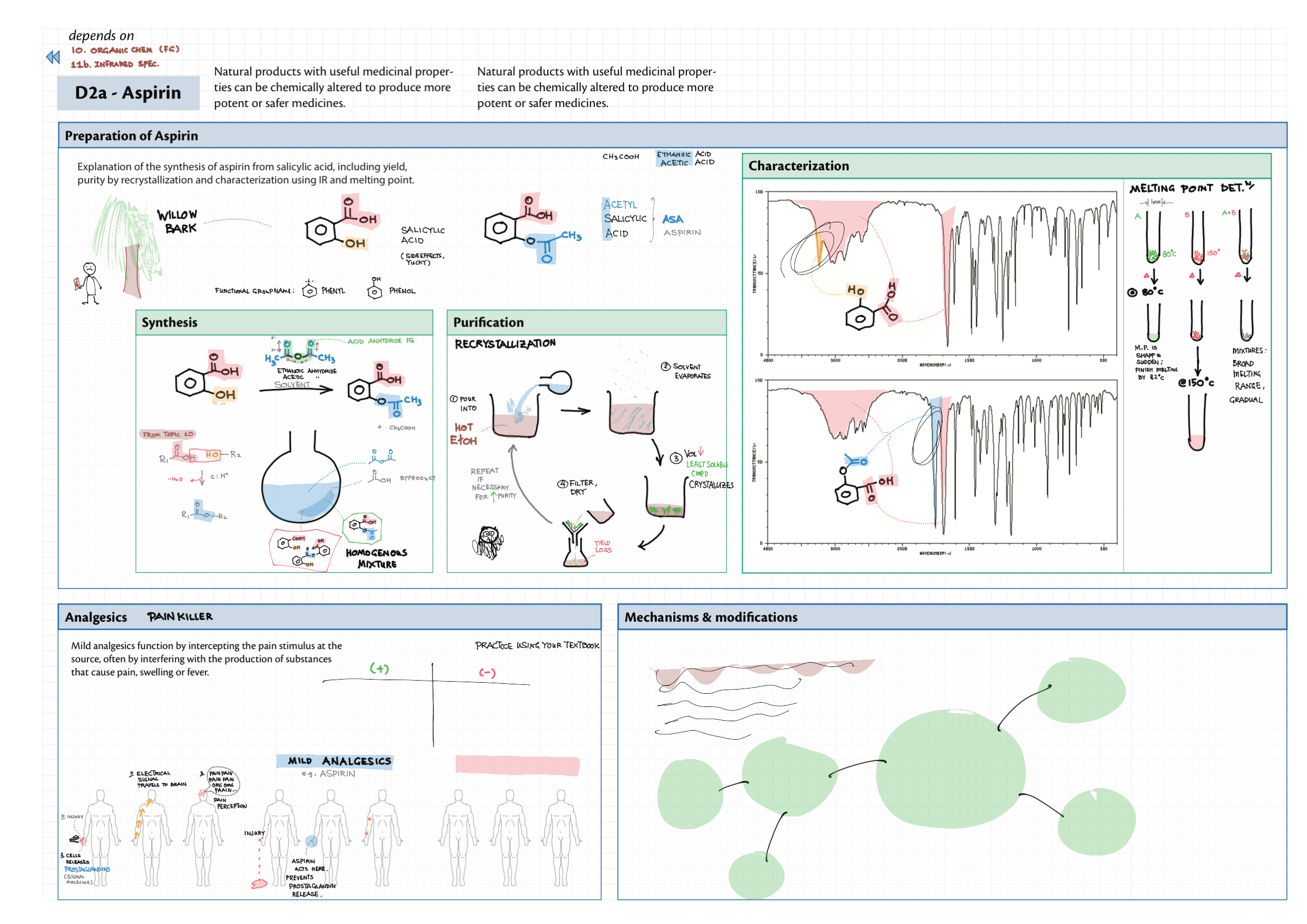
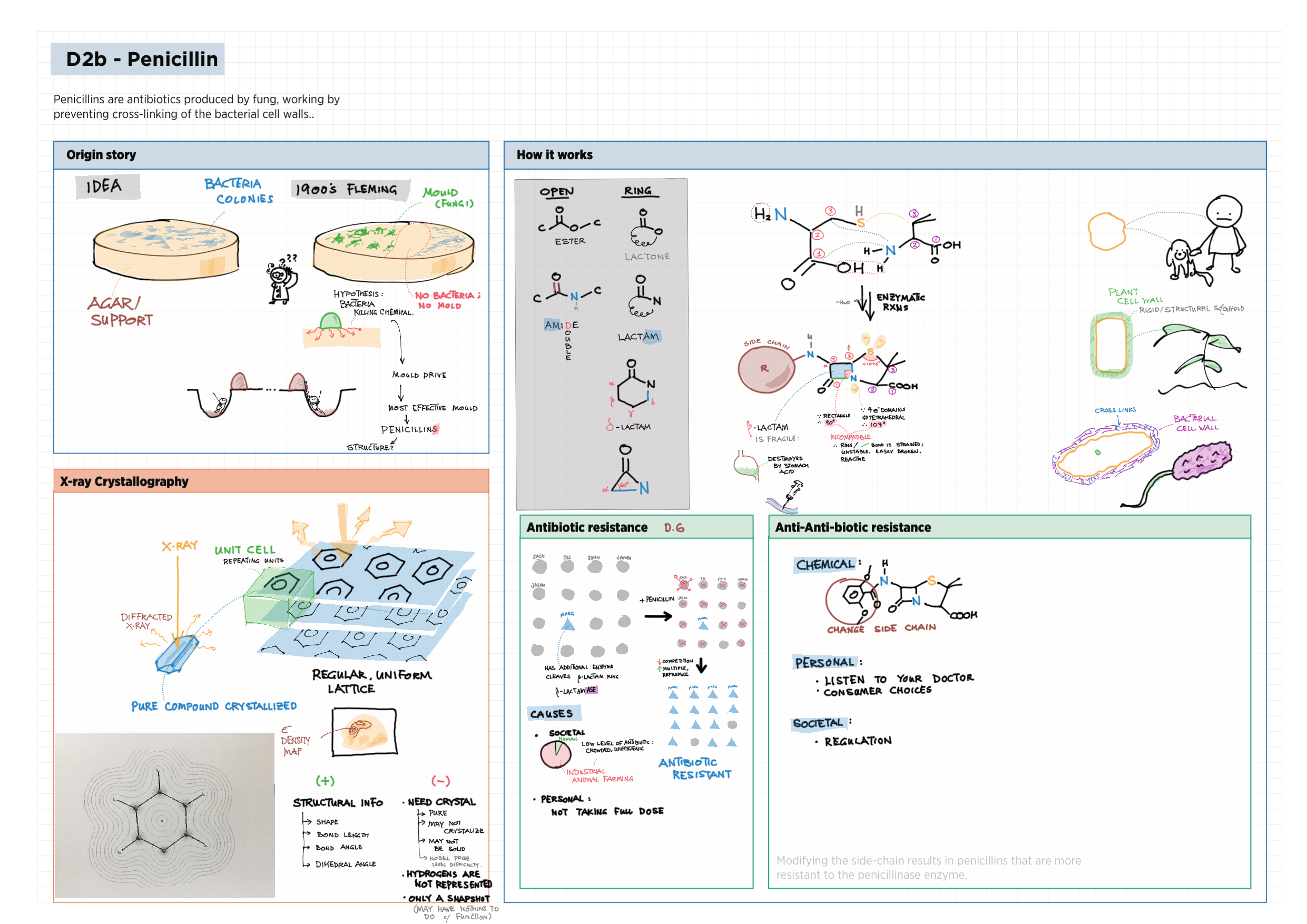
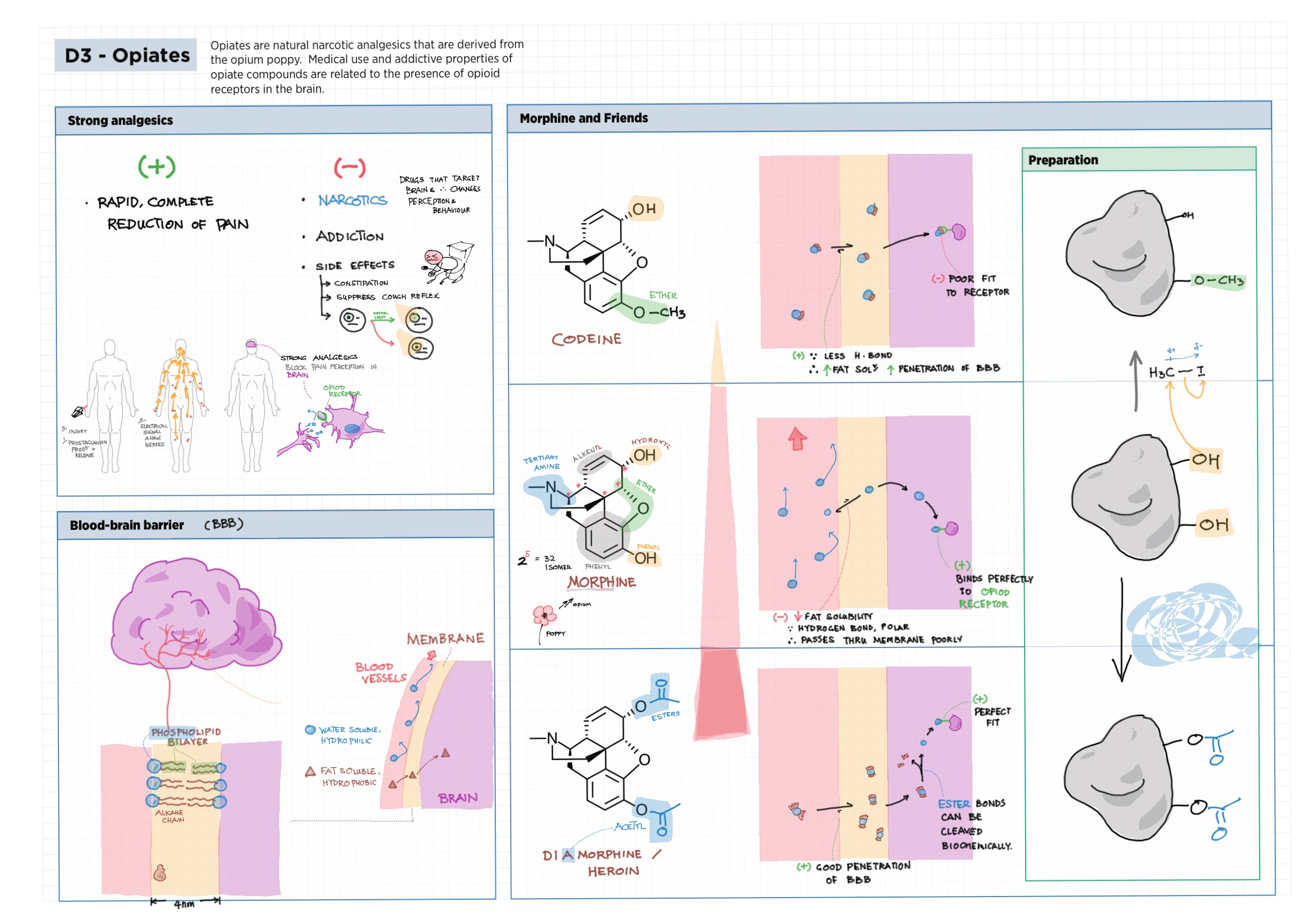
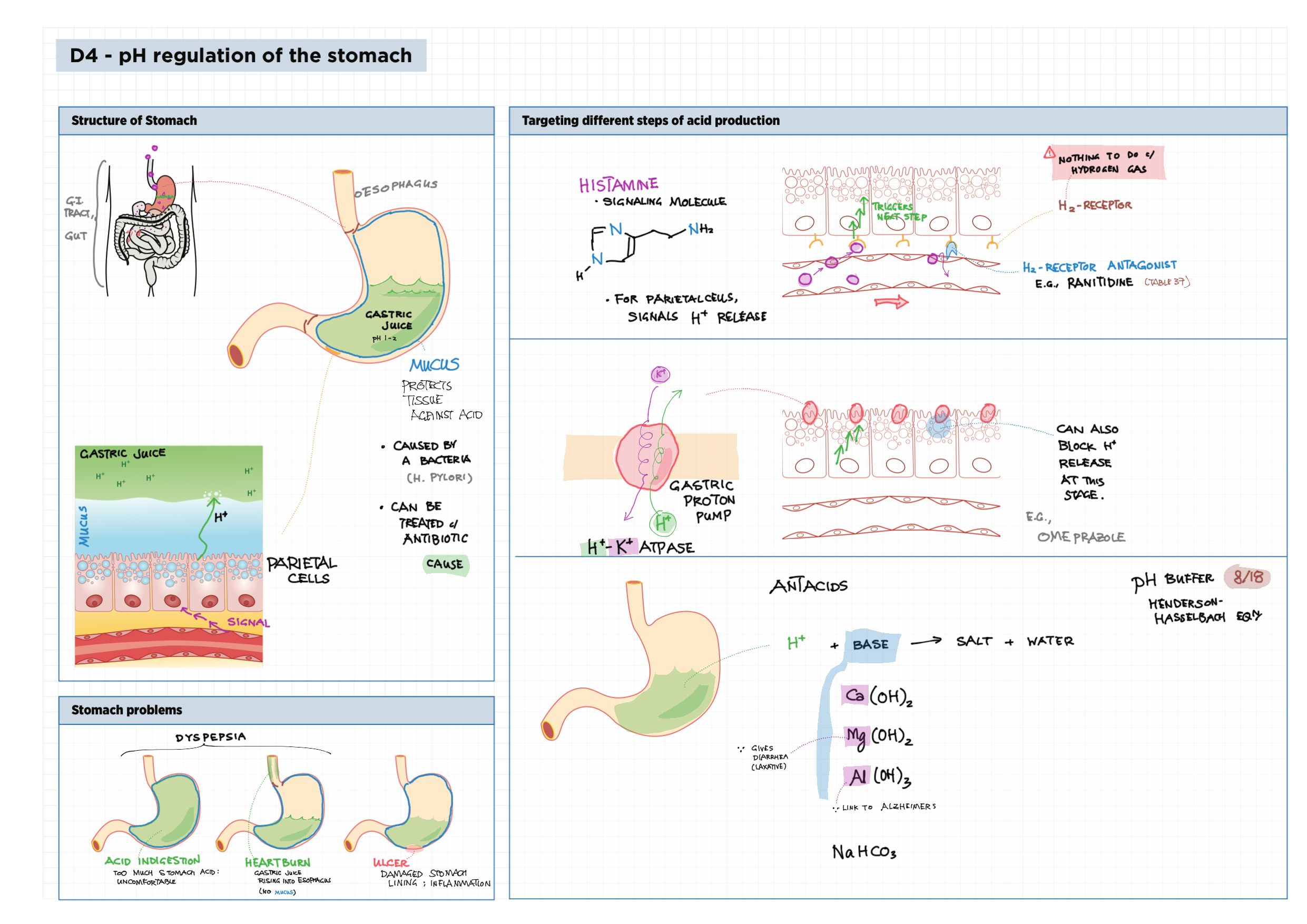
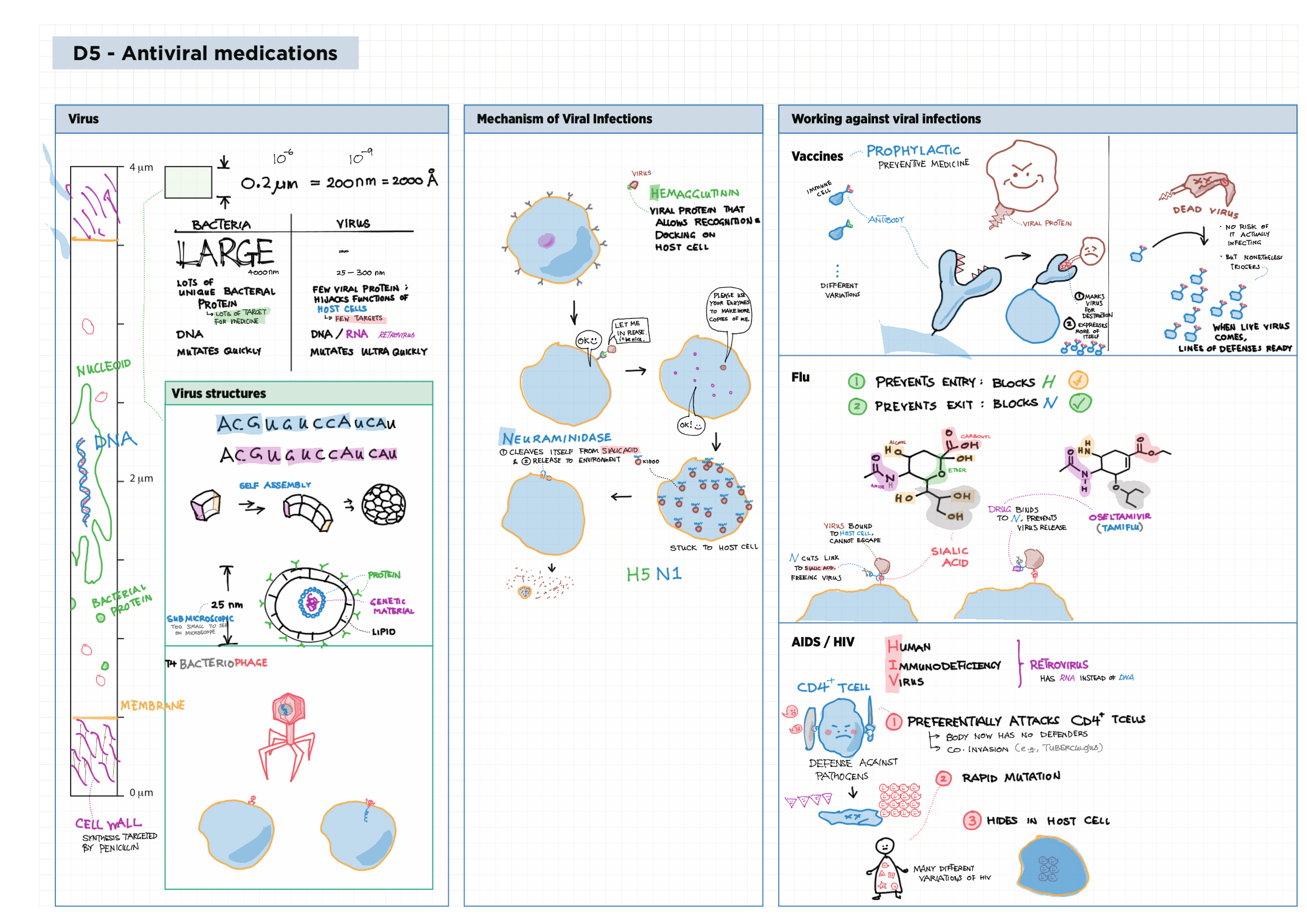
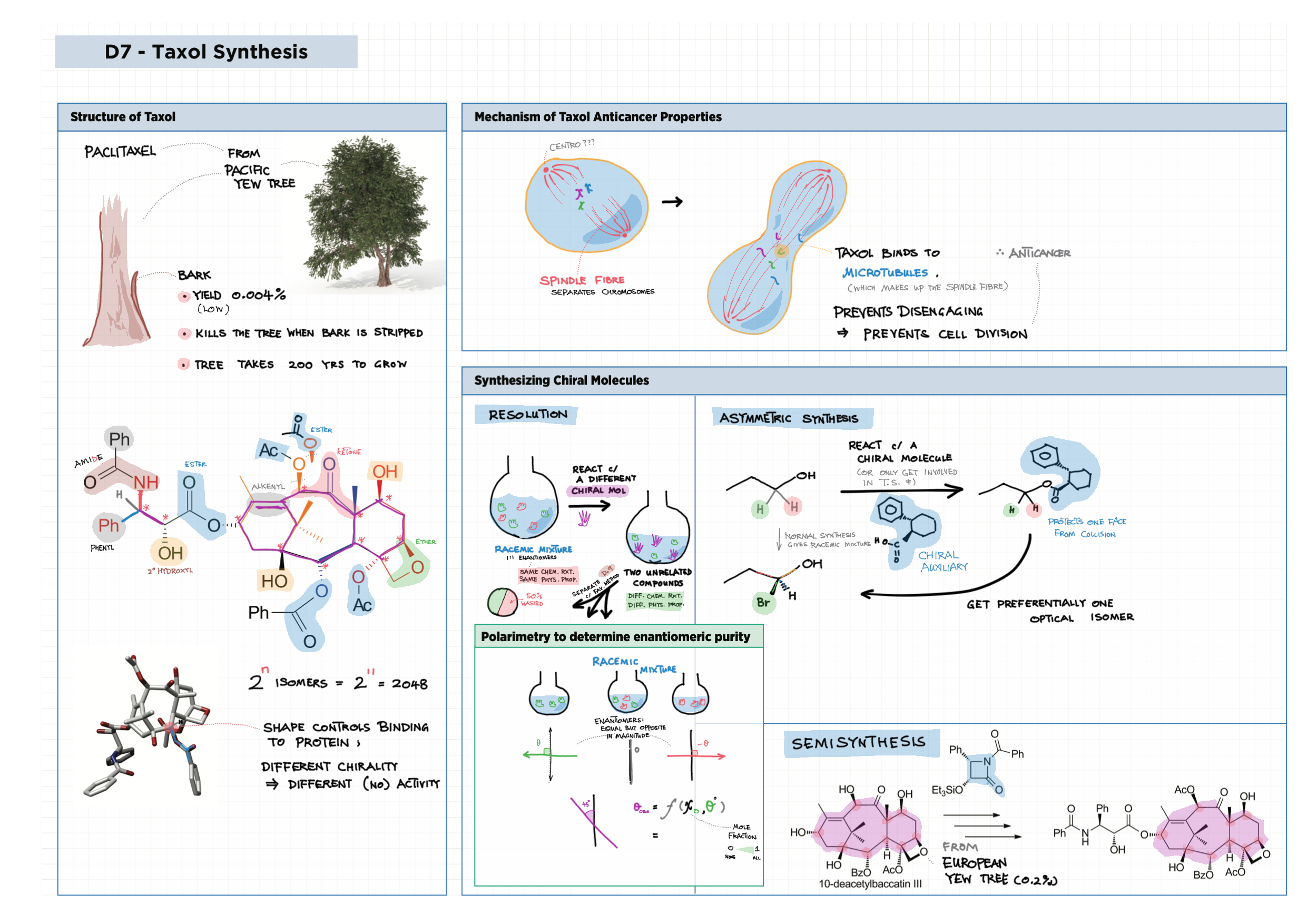
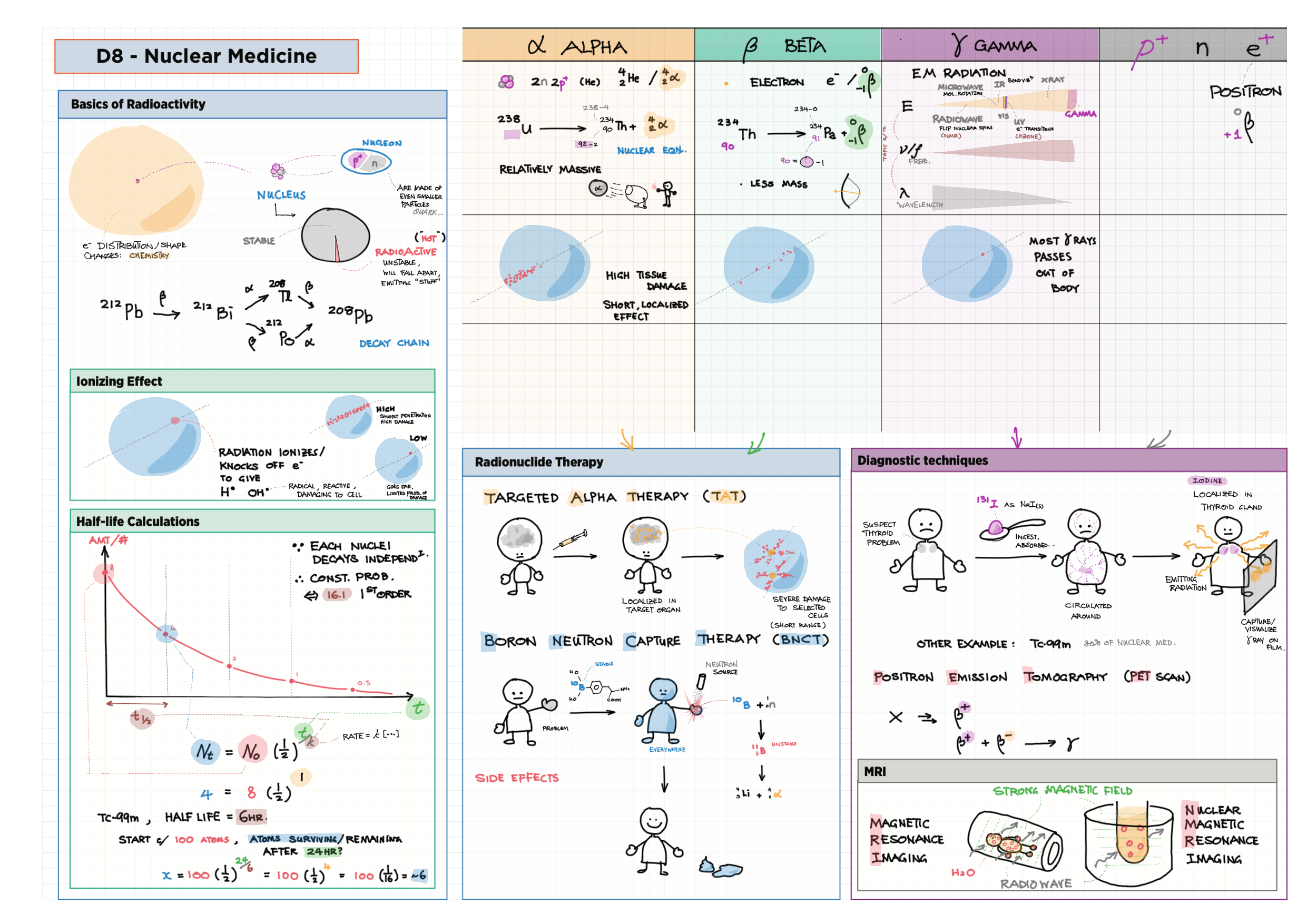
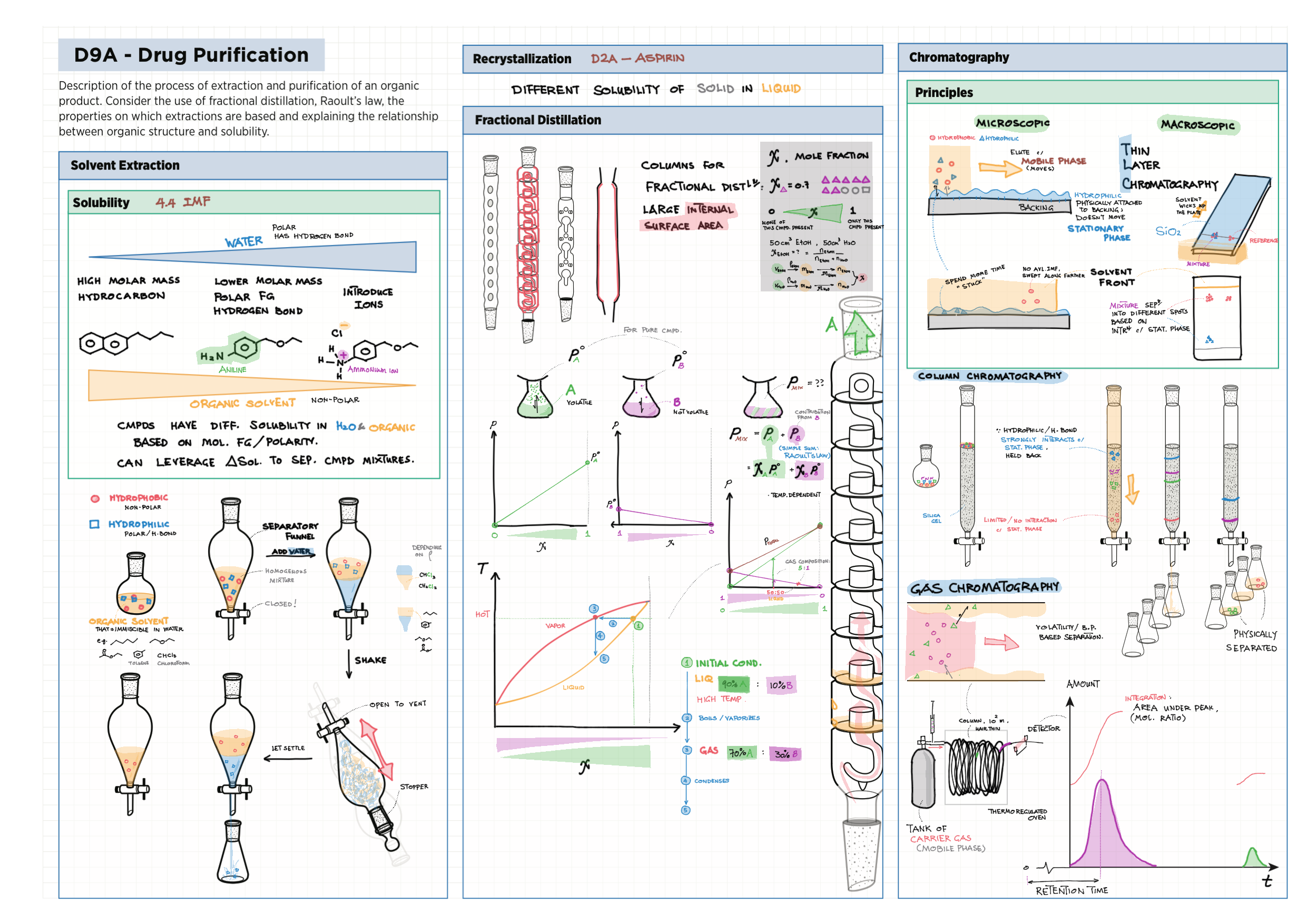
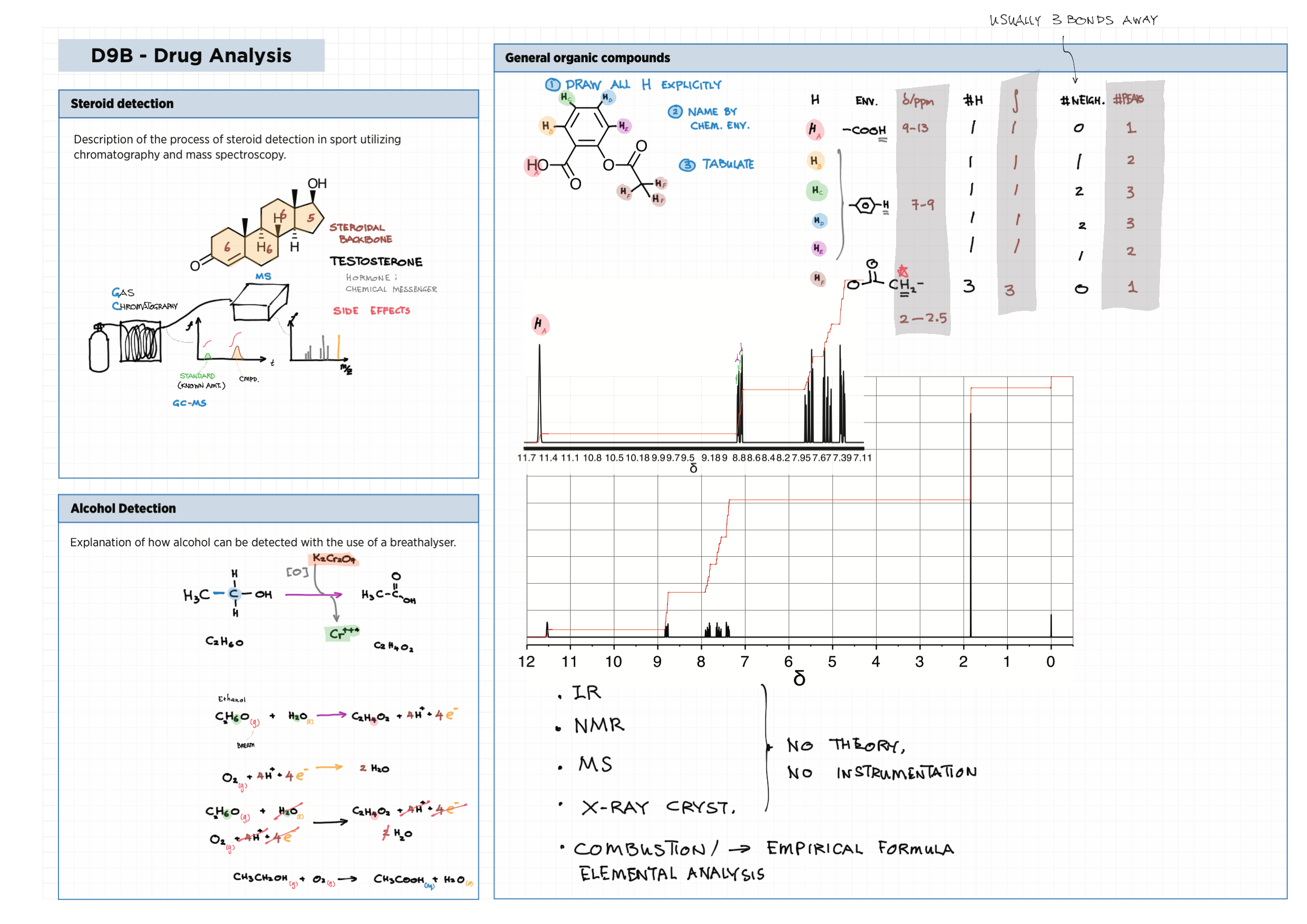
D - Medicinal Chemistry topics note D1 Med chem overview D2 Aspirin D2 / 21 Penicillin & X-ray crystallography D3 Opiates D4 pH regulation of stomach D5 Antivirals D6 Environmental considerations of Med Chem 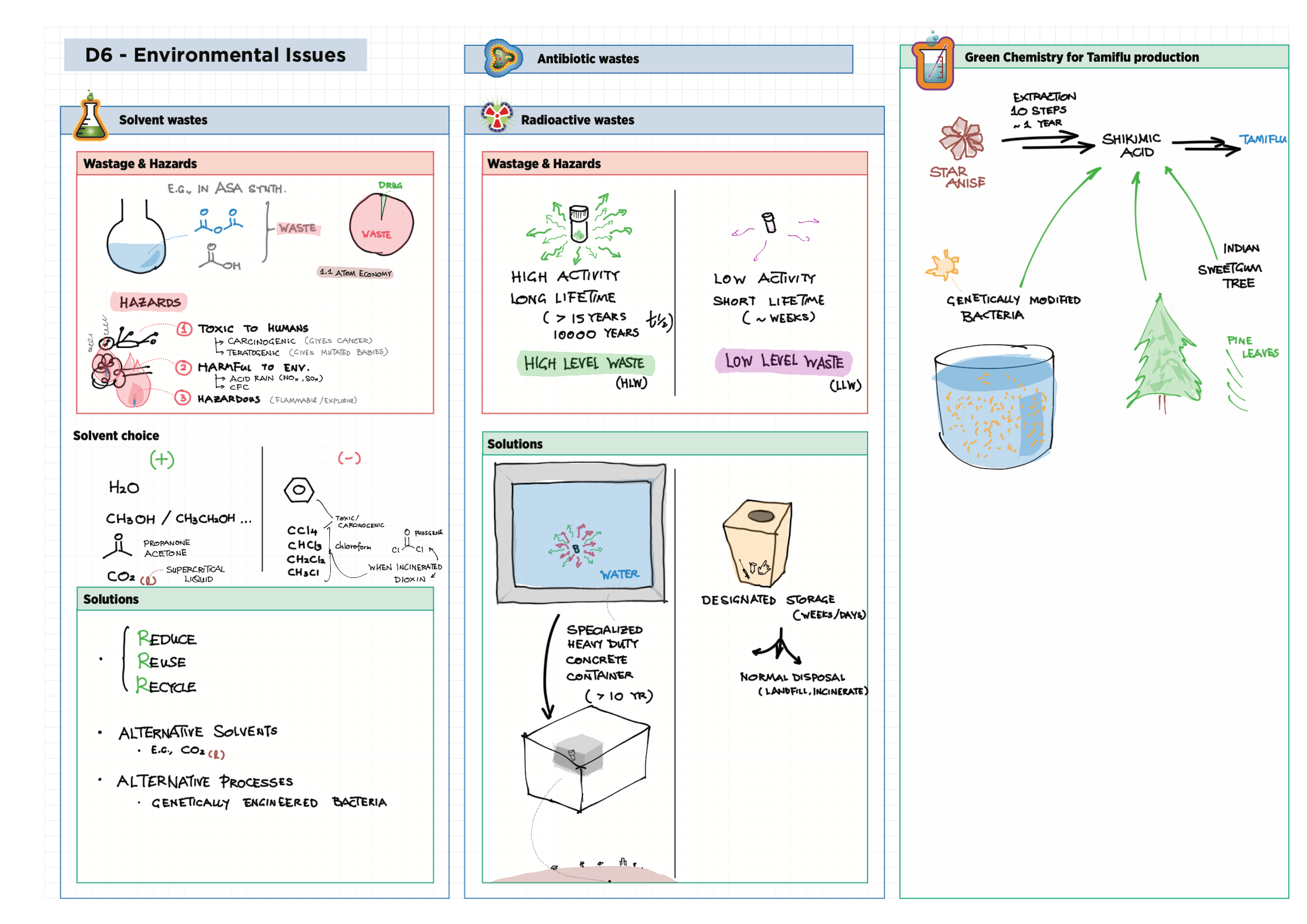 D7 (HL) Taxol D8 (HL) Nuclear medicine D9 (HL) Drug purification D9 (HL) Drug analysis
Last Updated: 2 years ago